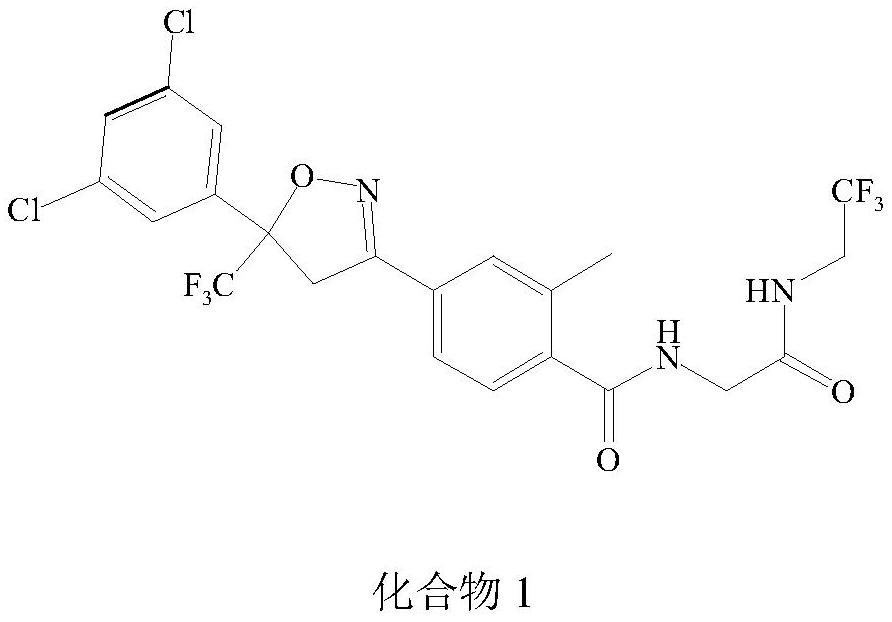
Preparation method of isoxazoline insectifuge
A technology for isoxazolines and anthelmintics, applied in the field of chemical drug synthesis, can solve problems such as increased cost, complicated operation, etc., and achieves the effects of saving raw materials, cheap raw materials, and short time consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
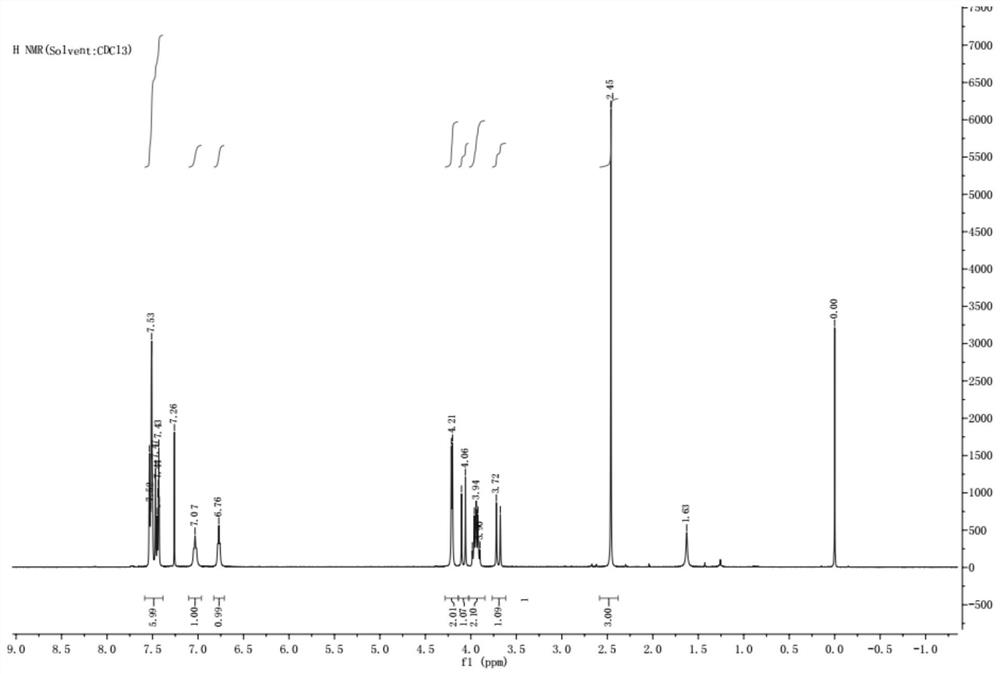
Embodiment 1
[0033] The first step: amidation condensation reaction
[0034]
[0035] Add 200ml of DMF to a 500ml four-neck flask, stir, then add 20g (0.11mol) of intermediate I, control the temperature at 20-30°C, add 18.0g (0.13mol) of HOBt, 25.6g (0.13mol) of EDC to the reaction solution Hydrochloride and 30g (0.16mol) intermediate II, then add 25.9g (0.26mol) Et 3 N, keep the reaction for 2 hours, use PE:EA=1:1 (volume ratio) developer to perform TLC detection, until the reaction of intermediate I is complete.
[0036] Step 2: Substitution Reaction
[0037]
[0038] Then 19.4g (0.15mol) NCS was slowly added in batches to the above reaction solution, and after the addition was completed, the reaction was incubated at 20-30°C for 2 hours. TLC (PE:EA=1:1) detected that the reaction of product 1 was complete.
[0039] The third step: ring closing reaction
[0040]
[0041] 32.2 g (0.13 mol) of intermediate III was added to the reaction solution obtained in the second step, and ...
Embodiment 2
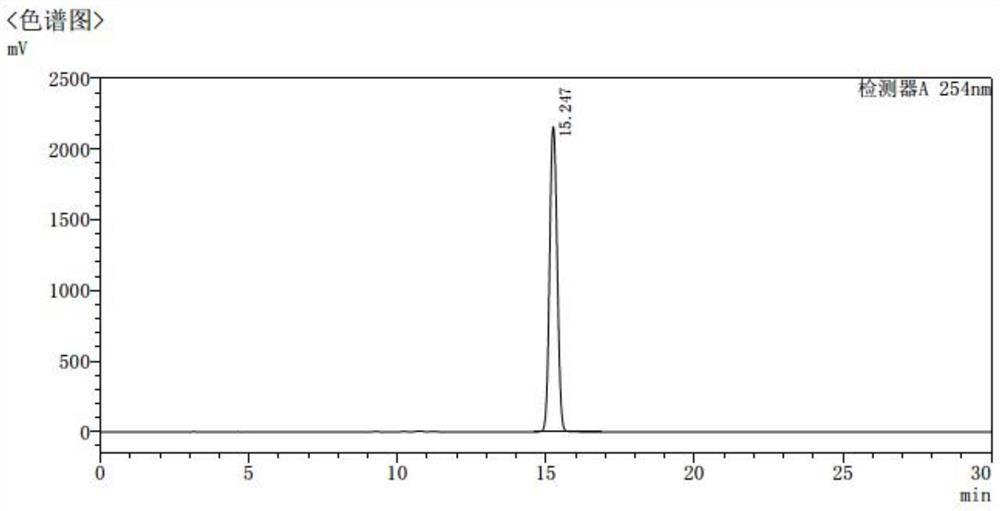
[0050] Add 200ml of DMSO to a 500ml four-neck flask, stir, then add 20g (0.11mol) of intermediate I, control the temperature at 20-30°C, add 18.0g (0.13mol) of HOBt, 25.6g (0.13mol) of EDC salt into the reaction solution salt and 30g (0.16mol) of intermediate II, then add 25.9g (0.26mol) of Et 3 N, keep the reaction for 2 hours, TLC detection (PE:EA=1:1) until the reaction of intermediate I is complete. Then slowly add 19.4 g (0.15 mol) NCS to the reaction liquid in batches, keep the reaction at 20-30° C. for 2 hours after the addition, and detect by TLC (PE:EA=1:1). Add 32.3g (0.13mol) of intermediate III to the reaction liquid, keep the reaction for 3 hours, and detect by TLC (PE:EA=1:1).
[0051] The reaction solution was slowly added to 2.8 L of purified water, stirred while adding, stirred for 1 hour after the addition, and filtered. The solid was recrystallized from petroleum ether and ethyl acetate, filtered, and air-dried to obtain 44 g, with a molar yield of 79.1%, ...
Embodiment 3
[0053] Add 200ml of DMF to a 500ml four-neck flask, stir, then add 20g (0.11mol) of intermediate I, and control the temperature at 20-30°C, add 37.8g (0.28mol) of HOBt, 53.48g (0.28mol) of EDC salt into the reaction solution salt and 32.6g (0.17mol) of intermediate II, then add 39.59g (0.39mol) of Et 3 N, keep the reaction for 2 hours, TLC detection (PE:EA=1:1) until the reaction of intermediate I is complete. Then slowly add 22.4g (0.17mol) NCS to the reaction liquid in batches, keep the reaction at 20-30°C for 2 hours after the addition, and detect by TLC (PE:EA=1:1). Add 40.5 g (0.17 mol) of intermediate III to the reaction liquid, keep the reaction for 3 hours, and detect by TLC (PE:EA=1:1).
[0054] The reaction solution was slowly added to 2.8 L of purified water, stirred while adding, stirred for 1 hour after the addition, and filtered. The solid was recrystallized from toluene and ethyl acetate, filtered, and air-dried to obtain 42.6 g, with a molar yield of 76.5%, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com