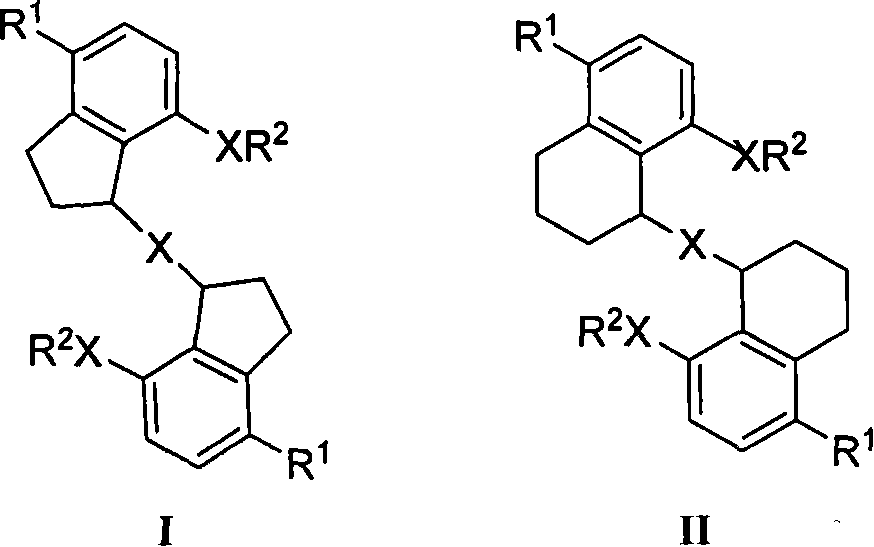
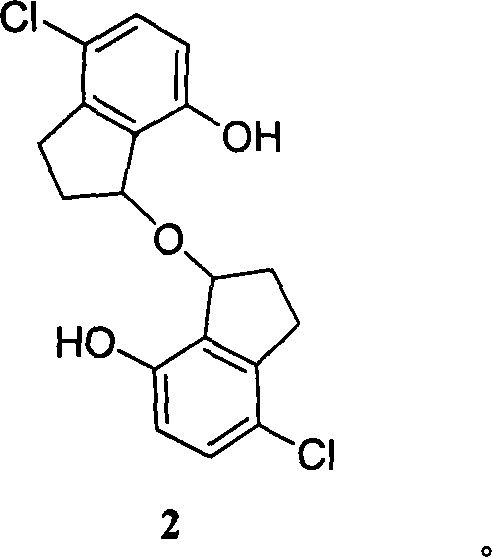
Di(7-hydrxyl-2,3-dihydro-1-1H-indeno)ether and the like, synthetic method and application
An analogue, dihydro technology, applied in the field of drug synthesis, can solve the problems of unsatisfactory effect of interferon hepatitis C, high production cost, long course of treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
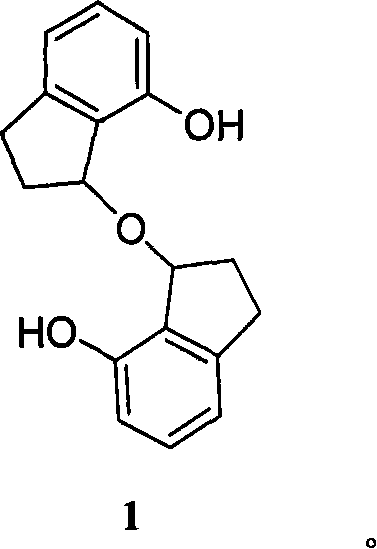
[0027] Embodiment 1: Starting from phenol to synthesize self-etherification product 1 (Scheme 1).
[0028]
[0029] Scheme 1.Synthesis of 1: (i) acryloyl chloride, Et 3 N, CH 2 Cl 2 , rt, 2h; (ii) AlCl 3 , 150-160°C, 6h, 15% (total yield of step i and ii); (iii) NaBH 4 / CH 3 OH, 75%; (iv) CH 2 Cl 2 , rt, one week, 35%.
Embodiment 2
[0031] Embodiment 2: the synthesis of phenol acrylate (3)
[0032] Phenol (1 g, 10.6 mmol) was dissolved in 5 mL of anhydrous CH 2 Cl 2 , cooled to 0°C in an ice-water bath, triethylamine (1.72mL, 11.7mmol) was added by injection, and acryloyl chloride (0.95mL, 11.7mmol) was slowly added dropwise in 2mL of anhydrous CH 2 Cl 2 Solution, a large amount of white smoke will be generated when dripping, after dripping, use 3ml of anhydrous CH 2 Cl 2 Rinse the buret. React at room temperature for 2 h, TLC showed that the raw materials basically disappeared, add 10 mL of saturated saline, stir for half an hour, CH 2 Cl 2 Extracted four times, the organic phase with anhydrous MgSO 4 dry. The desiccant was filtered, and the solvent was evaporated under reduced pressure to obtain a yellow viscous oil (3), which was drained and set aside.
Embodiment 3
[0033] Example 3: Synthesis of 7-hydroxyl-2,3-dihydroindan-1-one (4)
[0034]Add compound 3 and 11 g of anhydrous AlCl in a balloon-protected closed system 3 , the oil bath was heated to 150-160°C, and the reactant smoked violently. After reacting for 6 hours, cool to room temperature, and slowly add 2M hydrochloric acid dropwise to quench the reaction under cooling in an ice-water bath. During this process, the exothermic heat is violent until the solid basically dissolves and turns into a black solution. Ethyl acetate was extracted four times until TLC of the extract showed no product point. Combined organic phases, anhydrous MgSO 4 dry. TLC showed raw material (R f =0.83, developer: petroleum ether / ethyl acetate=10:1) has all disappeared, and there are two new spots (R f = 0.41 and R f = 0.22) generated. The desiccant was filtered, the solvent was evaporated under reduced pressure, and the residue was subjected to silica gel column chromatography separation, eluent: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com