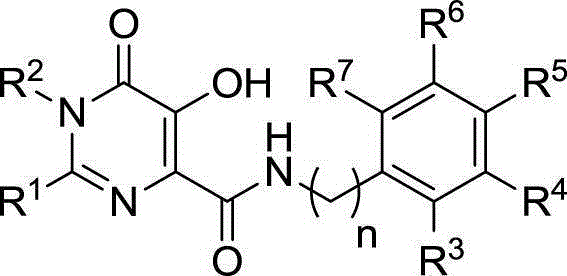
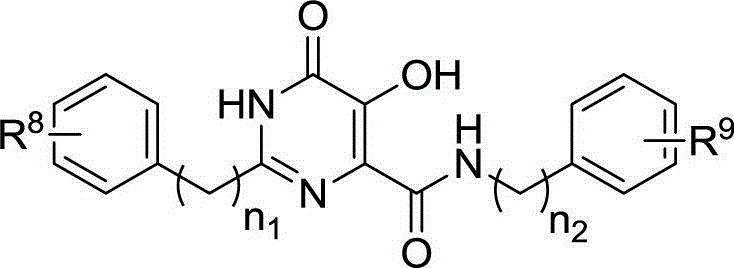
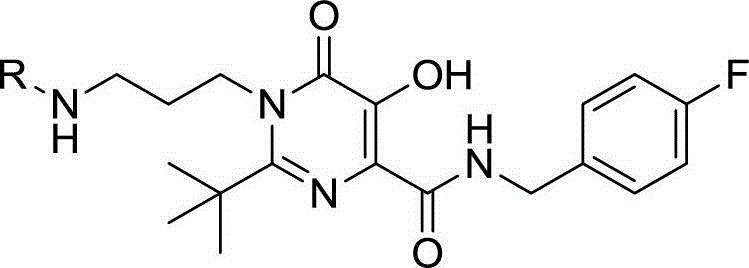
Hydroxypyrimidinone compounds and preparation method and application thereof
A technology of hydroxypyrimidinone and compound, applied in the field of hydroxypyrimidinone compounds, can solve the problems of emergence of drug-resistant virus, toxic and side effects, inability to clear virus and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] The preparation of embodiment 1. compound 5a~5t
[0093] (1) Preparation of substituted benzamide oxime or substituted phenylacetamide oxime 2
[0094] Put hydroxylamine hydrochloride (200mmol) and potassium carbonate (200mmol) in 200mL ethanol and stir at room temperature for 30 minutes. Substituted benzonitrile or phenylacetonitrile was added, the reaction solution was refluxed for 12 hours, filtered to remove inorganic salts, and the solvent was evaporated under reduced pressure. Compound 2 was obtained by silica gel column chromatography, and the elution system was petroleum ether / ethyl acetate 2:1 volume ratio.
[0095] The above-mentioned substituted benzonitriles or phenylacetonitriles are respectively selected from benzonitrile, p-fluorobenzonitrile, 2-fluorobenzonitrile, 3-fluorobenzonitrile, p-chlorobenzonitrile, p-bromoxynil, and p-fluorobenzonitrile to obtain the following intermediates respectively Compound 2.
[0096] 2a: 4-fluorobenzamide oxime, white s...
Embodiment 2
[0139] Example 2. Preparation of Compounds 14, 15, 16a~16n, 17a, 17c, 17d, 17f, 17g
[0140] (1) Preparation of trimethylacetamide oxime 6
[0141] Put hydroxylamine hydrochloride (200mmol) and potassium carbonate (200mmol) in 200mL ethanol and stir at room temperature for 30 minutes. Trimethylacetonitrile 6 (75 mmol) was added, the reaction solution was refluxed for 12 hours, filtered to remove inorganic salts, the solvent was evaporated under reduced pressure, and the obtained residue was recrystallized with ether / petroleum ether to obtain compound 7.
[0142] 7: Trimethylacetamide oxime, white solid, yield 76.5%, mp: 119.4~120.6°C. 1 HNMR (DMSO-d 6 )δ (ppm): 8.86 (s, 1H), 5.22 (s, 2H), 1.08 (s, 9H).
[0143] (2) Preparation of dimethyl 2-(((1-amino-2,2-dimethylpropylene)amino)hydroxy)maleate 8
[0144] Trimethylacetamide oxime 7 (20 mmol) was dissolved in 50 ml of dry methanol. 1.2 times the amount of dimethyl butynedioate (24 mmol) was added under ice-bath conditions....
Embodiment 3
[0188] Example 3. Determination of compound's inhibitory activity on HIV-1 integrase, antiviral activity and cytotoxicity.
[0189] Compounds 5a-5t, 14, 15, 16a-16n, 17a, 17c, 17d, 17f, 17g prepared in Examples 1 and 2 were tested for biological activity according to the following method, and the results are listed in Table 1 and Table 2.
[0190] The inhibitory activity of the compound on HIV-1 integrase, the assay method of antiviral activity and cytotoxicity, adopt the method reported in the literature, see for details:
[0191] S. Yu, L. Zhang, S. Yan, P. Wang, T. Sanchez, F. Christ, Z. Debyser, N. Neamati, G. Zhao, Nitrogen-containing polyhydroxylated aromatics as HIV-1 integrase inhibitors: synthesis, structure-activity relationship analysis, and biological activity, JEnzyme Inhib MedChem, 27 (2012) 628-640.
[0192] Table 1. Compounds 5a~5t have HIV-1 integrase inhibitory activity, antiviral activity and cytotoxicity assay data
[0193]
[0194]
[0195]
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com