Preparation method of hepatitis C and novel coronavirus drug intermediate and salt thereof
A technology for hepatitis C and intermediates, applied in the field of drug synthesis, can solve the problems of poor reaction selectivity, high production cost, low yield and the like, and achieve the effects of short reaction steps, short production time, and easily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
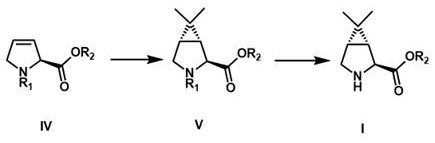
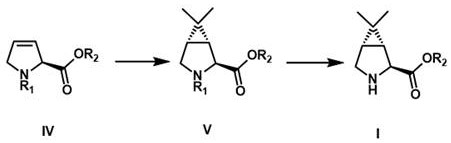
[0041] Embodiment 1: (1 R ,2 S ,5 S )- N Synthesis of -tert-butoxycarbonyl-6,6-dimethyl-3-azabicyclo[3,1,0]hexyl-2-carboxylic acid methyl ester
[0042]
[0043] (S)- N -tert-butoxycarbonyl-2,5-dihydro-1 H -Methyl pyrrole-2-carboxylate can be conveniently prepared in high yield by a published method (document US2008 / 0027262).
[0044] Under nitrogen protection, the (S)- N -tert-butoxycarbonyl-2,5-dihydro-1 H -Methyl pyrrole-2-carboxylate (1.00 eq, 34.2 g) was dissolved in 800 mL of dry dichloromethane, cooled to –78 °C in a dry-ice acetone bath, and then a diethyl ether solution of 2-diazopropane was added dropwise (according to literature Org. Synth., 1970, 50, 27. Preparation) until the conversion of raw materials monitored by TLC was complete. After the system was heated to 20-25 °C to continue the reaction for 2 h, the solvent was concentrated and replaced with methyl tert-butyl ether, and then cooled in an ice-water bath. Maintain at 0-5°C, irradiate with a 30...
Embodiment 2
[0045] Embodiment 2: (1 R ,2 S ,5 S )- N Synthesis of -Benzyloxycarbonyl-6,6-dimethyl-3-azabicyclo[3,1,0]hexyl-2-carboxylic acid methyl ester
[0046]
[0047] (2 S ,4 R )- N -Benzyloxycarbonyl-4-hydroxyproline methyl ester can be conveniently prepared in high yield by the disclosed method (document CN112930350A).
[0048] Under nitrogen protection, 48.1 g of 1-propyl cyclophosphoric anhydride in ethyl acetate solution (50% w / w, 1.32 eq.) was added to the reactor, cooled to 0 °C under stirring, and 15.9 g ( 2 S ,4 R )- N - Benzyloxycarbonyl-4-hydroxyproline methyl ester (1.00 eq.) in 50 mL of ethyl acetate. After the dropwise addition, the reaction was incubated until the reaction of the raw materials was monitored by TLC, and the reaction solution was washed with 50 mL of water. The organic phase was dried over anhydrous sodium sulfate and concentrated to obtain compound (S)- N -Benzyloxycarbonyl-2,5-dihydro-1 H - Methyl pyrrole-2-carboxylate.
[0049] Under ni...
Embodiment 3
[0050] Embodiment 3: (1 R ,2 S ,5 S )- N Synthesis of -tert-butoxycarbonyl-6,6-dimethyl-3-azabicyclo[3,1,0]hexyl-2-carboxylic acid methyl ester
[0051]
[0052] Under nitrogen protection, add (S)- N -tert-butoxycarbonyl-2,5-dihydro-1 H - Methyl pyrrole-2-carboxylate (1.00 eq., 11.5 g) and 2,2-dibromopropane (3.00 eq., 30.6 g), cooled to -78 °C in a dry ice acetone bath. A solution of n-butyllithium in n-hexane (2.00 eq.) was then slowly added dropwise. After the dropwise addition was completed, the temperature was raised to 20-25° C. to continue the reaction for 12 hours, then a saturated ammonium chloride solution was added, and the organic phase was washed with water and dried over anhydrous sodium sulfate, concentrated to remove the solvent and used column chromatography (petroleum ether / acetic acid Ethyl ester) was purified to obtain slightly oily compound (1 R ,2 S ,5 S )- N - methyl tert-butoxycarbonyl-6,6-dimethyl-3-azabicyclo[3,1,0]hexyl-2-carboxylate (6....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com