Synthesis method of dapoxetine hydrochloride
A technology of dapoxetine hydrochloride and a synthesis method, applied in the field of synthesis of dapoxetine hydrochloride, can solve the problems of high production cost, low yield, expensive chiral resolving agent and the like, and achieves the reaction time Short, high product yield, easy post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
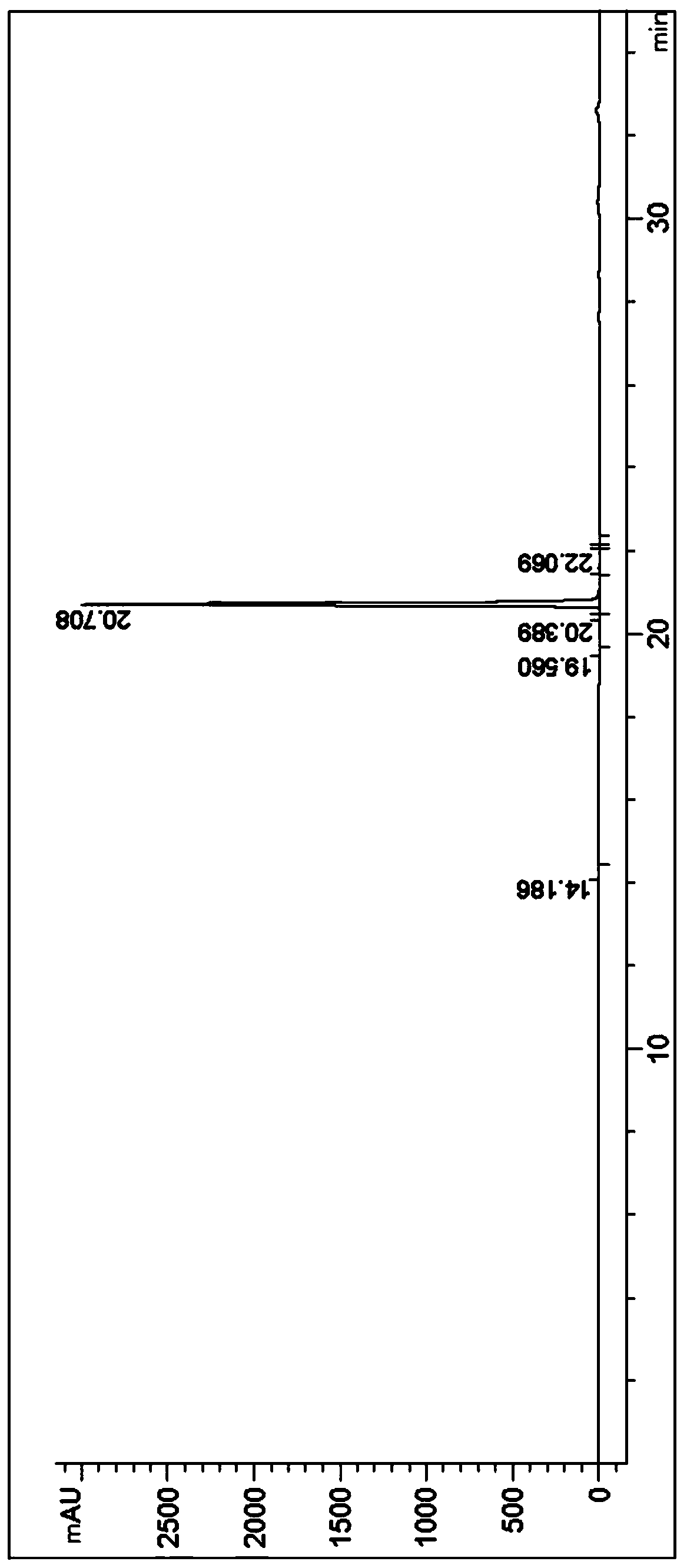
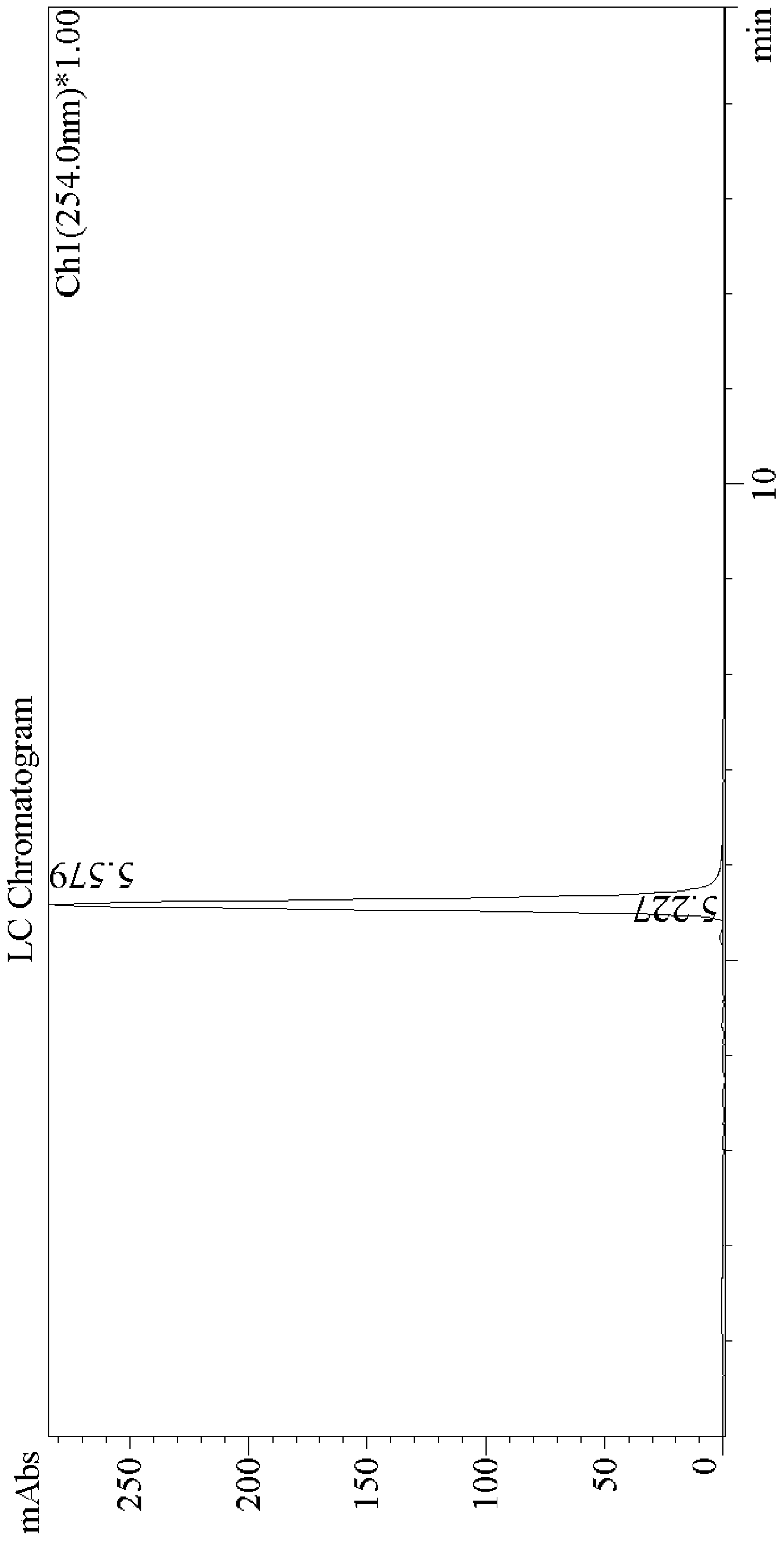
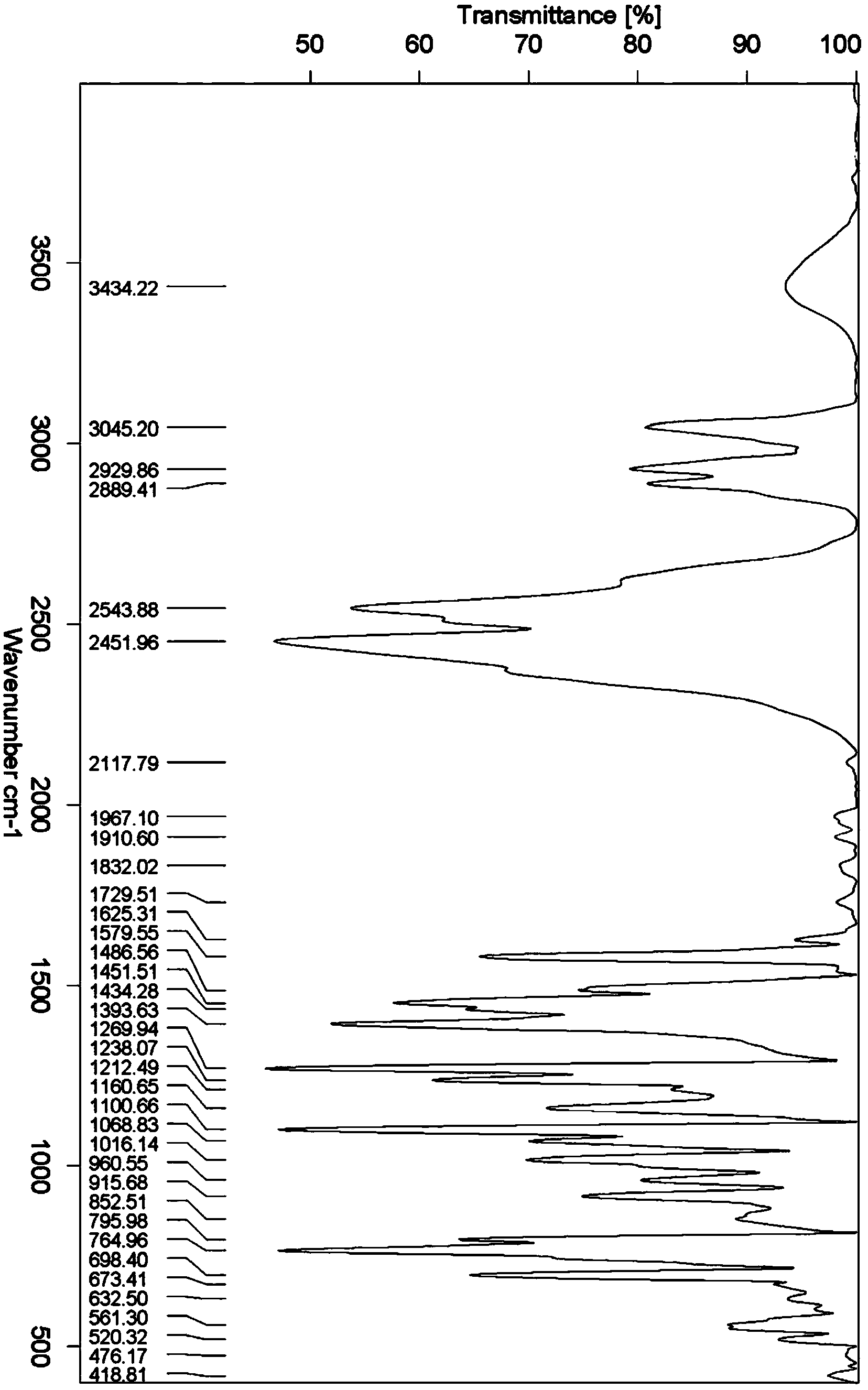
Image
Examples
Embodiment 1
[0038] Disperse 1300g of (S)-3-amino-3-phenylpropionic acid in 13L of tetrahydrofuran, add 964g of sodium borohydride in 3 batches while stirring under the protection of nitrogen at 10-20°C, and continue stirring to cool down after adding Add 1250g of concentrated sulfuric acid (98%) dropwise at 8°C, and finish the dropwise addition in about 4 hours, then naturally rise to room temperature and stir for 12 hours until TLC detects that the raw material disappears, then slowly add 100mL of methanol at 45°C to quench the reaction and remove Add excess sodium borohydride, then add 20% NaOH aqueous solution to adjust the pH of the reaction solution to 12, heat to reflux for 2 hours, and after cooling to room temperature, extract the reaction solution three times with 10L toluene, combine the toluene layers, and concentrate under reduced pressure to obtain a colorless Oil (S)-3-amino-3-phenylpropanol.
[0039]Dissolve the (S)-3-amino-3-phenylpropanol obtained above in 1.916kg85% form...
Embodiment 2
[0042] Disperse 1400g of (S)-3-amino-3-phenylpropionic acid in 14L of methyl tert-butyl ether, add 1464g of potassium borohydride in 3 batches while stirring under nitrogen protection at 10°C, after the addition is complete Continue to stir and drop 816g of acetic acid when the temperature is lowered to 5°C, and add dropwise in about 4 hours. After the dropping, naturally rise to room temperature and stir for 12 hours until TLC detects that the raw material disappears, then slowly add 120mL of saturated ammonium chloride aqueous solution at 40°C to quench React to remove excess potassium borohydride, then add 20% KOH aqueous solution to adjust the pH of the reaction solution to 12, heat to reflux for 2 hours, and after cooling to room temperature, extract the reaction solution three times with 12L ethyl acetate respectively, after merging the ethyl acetate layers, Concentration under reduced pressure gave (S)-3-amino-3-phenylpropanol as a colorless oil.
[0043] Dissolve the (...
Embodiment 3
[0046] Disperse 1500g of (S)-3-amino-3-phenylpropionic acid in 15L of diethyl ether, add 630g of lithium borohydride in 3 batches while stirring under the protection of nitrogen at 20°C, continue to stir and cool down to 10 Add 1859g of aluminum trichloride at ℃, and finish adding in about 4 hours. After dropping, naturally rise to room temperature and stir for 12 hours until TLC detects that the raw material disappears. Slowly add 100mL of 1N dilute hydrochloric acid at 50℃ to quench the reaction to remove excess lithium borohydride , then add 20% NaOH aqueous solution to adjust the pH of the reaction solution to 13, heat and reflux for 3 hours, and after cooling to room temperature, extract the reaction solution three times with 14L ethyl acetate respectively, after combining the ethyl acetate layers, concentrate under reduced pressure to obtain a colorless oil (S)-3-amino-3-phenylpropanol.
[0047] Dissolve the (S)-3-amino-3-phenylpropanol obtained above in 2.1917kg85% form...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com