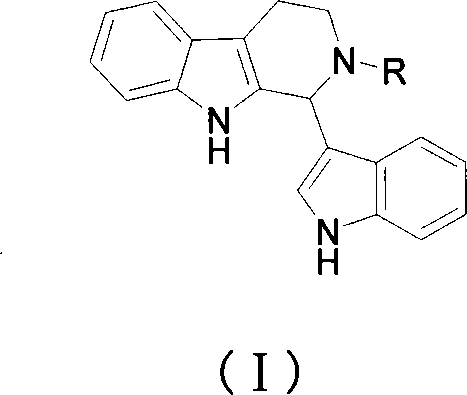
1-(3-indolyl)-1,2,3,4-tetrahydro-beta-carboline derivative and its prepn and use
A technology of indolyl and derivatives, applied in botany equipment and methods, chemicals for biological control, biocides, etc., can solve the problems of anti-tumor drugs that cannot meet the needs of treatment, chemotherapy failure, tumor cell growth, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
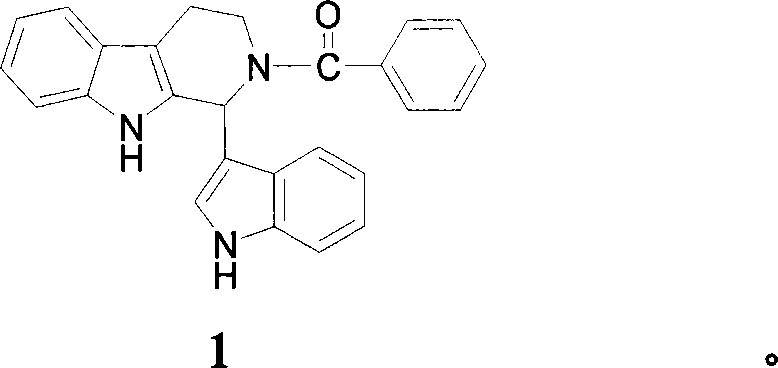
[0091] Embodiment 1: synthetic compound 1,1-(3-indolyl)-2-benzoyl-1,2,3,4-tetrahydro-β-carboline 1) Preparation of indole-3-formaldehyde
[0092] Put 56ml of DMF into a 500ml three-neck flask, stir in an ice-salt bath, keep the temperature not exceeding 0°C, and add 17.4ml (0.186mol) of phosphorus oxychloride dropwise. Then slowly add 20 ml of DMF solution containing 20 g (0.171 mol) of indole dropwise, and finish dropping within 1 hour. After the dropwise addition, the temperature of the reaction solution was kept at 35° C. for 1.5 hours. Under cooling in an ice-water bath, add 80ml of ice water, then slowly add 200ml of aqueous solution containing 80g of sodium hydroxide (1 / 3 of the amount is slowly added first, and the rest is added at one time), heat and reflux for 15 minutes, cool and place in the refrigerator 4 hours. Suction filtration, the filter cake was washed with a large amount of water, after being drained, it was dried under an infrared lamp. 23.0 g of light y...
Embodiment 2
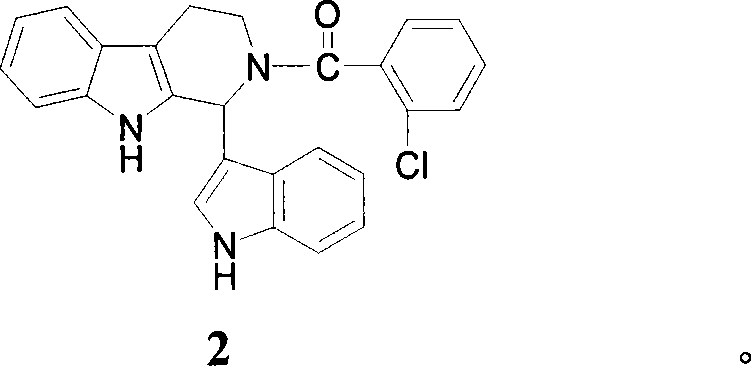
[0099] Example 2: Synthetic compound 2,1-(3-indolyl)-2-o-chlorobenzoyl-1,2,3,4-tetrahydro-β-carboline
[0100] 1-(3-indolyl)-1,2,3,4-tetrahydro-β-carboline reacts with o-chlorobenzoyl chloride, the synthesis process is the same as 1. Yield: 27.0%. 1 HNMR (CDCl 3 )δ (ppm): 2.76 (m, 1H, Carboline-C 4 -H), 3.12(m, 1H, Carboline-C 4 -H), 3.48(m, 2H, Carboline-C 3 -H), 6.88-7.75 (m, 14H, Ar-H), 8.09-8.33 (m, 2H, NH). MS (ESI) m / z (M-1) 424.1.
Embodiment 3
[0101] Example 3: Synthetic compound 3,1-(3-indolyl)-2-p-chlorobenzoyl-1,2,3,4-tetrahydro-β-carboline
[0102]1-(3-indolyl)-1,2,3,4-tetrahydro-β-carboline reacts with p-chlorobenzoyl chloride, the synthesis process is the same as 1. Yield: 60.8%. 1 HNMR (DMSO-d6) δ (ppm): 2.71-2.85 (m, 2H, Carboline-C 4 -H), 3.40(m, 1H, Carboline-C 3 -H), 3.55(m, 1H, Carboline-C 3 -H), 6.88(s, 1H, Carboline-C 1 -H), 6.95-7.03(m, 2H, Indole-C 6’ -H+Carboline-C 7 -H), 7.05-7.12(m, 2H, Indole-C 5’ -H+Carboline-C 6 -H), 7.20(s, 1H, Indole-C 2’ -H), 7.28(d, 1H, Indole-C 7’ -H, J=7.8Hz), 7.34-7.40 (m, 3H, Ar-H+Carboline-C 8 -H), 7.44-7.52(m, 3H, Ar-H+Carboline-C 5 -H), 7.63(d, 1H, Indole-C 4’ -H, J=7.8 Hz), 11.05-11.10 (m, 2H, NH). MS (ESI) m / z (M-1) 424.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com