Preparation method of hydrocortisone
A technology of hydrocortisone and calcium chloride, applied in the direction of organic chemistry, steroids, etc., can solve the problems of high acid corrosion, high energy consumption, large investment, etc., and achieve the effect of good quality and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] A preparation method of hydrocortisone, comprising the steps of:
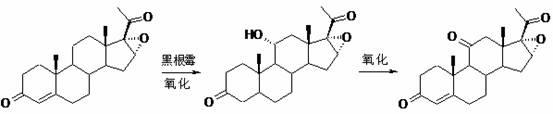
[0059] (1) Ketal reaction: Preparation of 17α-hydroxy-4-pregnene-11-one-3,20-diethylene glycol ketal (Ⅲ)
[0060] Put 50g of 17α-hydroxy-4-pregnene-3,11,20-trione (Ⅱ), 150ml of ethylene glycol and 150ml of triethyl orthoformate into the reaction flask, stir, and add 2.5g of p-toluenesulfonic acid , stirred and reacted at 30-35°C for 8 hours; after the reaction was completed, add 1500ml of water for water analysis, filter, wash with water until neutral, drain, and dry to obtain 17α-hydroxy-4-pregnene-11-one-3,20 - Diethylene glycol ketal (III) 60.8 g. MP: 228~233℃, [α] D 20 =-12.2° (10 mg / ml chloroform).
[0061] (2) Reduction reaction: Preparation of 11β,17α-dihydroxy-4-pregnene-3,20-diethylene glycol ketal (Ⅳ)
[0062] Put 50g of 17α-hydroxy-4-pregnene-11-one-3,20-diethylene glycol ketal (Ⅲ), 500ml of tetrahydrofuran, and 50ml of methanol into the reaction flask, stir, and slowly add 15g of sodium ...
Embodiment 2
[0073] Other operations are the same as embodiment 1, the difference is:
[0074] (2) Reduction reaction: Preparation of 11β,17α-dihydroxy-4-pregnene-3,20-diethylene glycol ketal (Ⅳ)
[0075] Put 50g of 17α-hydroxy-4-pregnene-11-one-3,20-diethylene glycol ketal (Ⅲ), 500ml of tetrahydrofuran, and 50ml of methanol into the reaction flask, stir, and slowly add 25g of potassium borohydride, Reflux for 48 hours; concentrate under reduced pressure to recover the solvent, add 1500ml of water for water analysis, filter, wash with water until neutral, drain and dry to obtain 11β,17α-dihydroxy-4-pregnene-3,20-diethylenedi Alcohol ketal (IV) 49.5g. MP: 219~225℃, [α] D 20 =-39.0° (10 mg / ml chloroform).
[0076] After testing, the MP of hydrocortisone prepared in this example: 215.5-219°C, [α] D 20 (Specific optical rotation) = +165.8° (10 mg / ml absolute ethanol), HPLC content: 98.7%, yield 77.1%.
Embodiment 3
[0078] Other operations are the same as embodiment 1, the difference is:
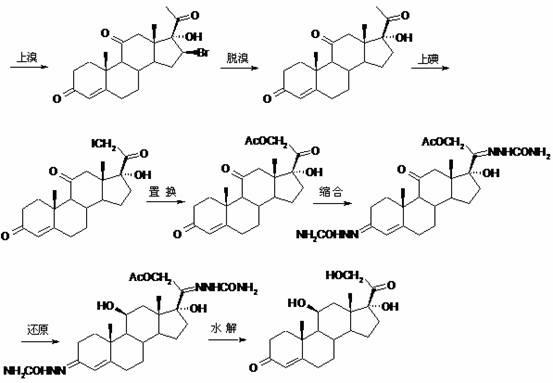
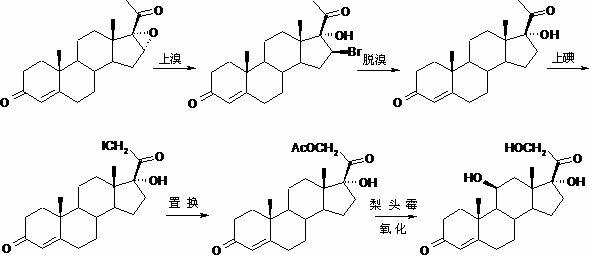
[0079] (3) Hydrolysis reaction: Preparation of 11β,17α-dihydroxy-4-pregnene-3,20-dione (Ⅴ)
[0080] In the reaction flask, put 50g of 11β,17α-dihydroxy-4-pregnene-3,20-diethylene glycol ketal (Ⅳ), 200ml of chloroform and 200ml of methanol, add 10ml of 50% sulfuric acid, and dissolve in 10 The reaction was stirred at ~20°C for 3 hours. After the reaction is completed, add ammonia water to neutralize, concentrate under reduced pressure to recover the solvent, concentrate until the dichloromethane is exhausted, add 1500ml of water for water analysis, filter, wash, drain, and dry to obtain 11β,17α-dihydroxy-4-pregnene- 3,20-diketone (Ⅴ) 38.2g. MP: 214~217.5℃, [α] D 20 =+152.6° (10mg / ml absolute ethanol).
[0081] After testing, the MP of hydrocortisone prepared in this example: 215-219°C, [α] D20 (Specific optical rotation) = +164.1° (10 mg / ml absolute ethanol), HPLC content: 98.5%, yield 77.8%.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com