Synthesis method of anti-influenza and avian influenza virus resistant medicine peramivir
A technology of avian influenza virus and synthesis method, which is applied in the field of organic drug synthesis and can solve the problems of environmental pollution, unapproved marketing, and long route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0064] The present invention will be further described in detail below in conjunction with the embodiments and the accompanying drawings, but the embodiments of the present invention are not limited thereto.
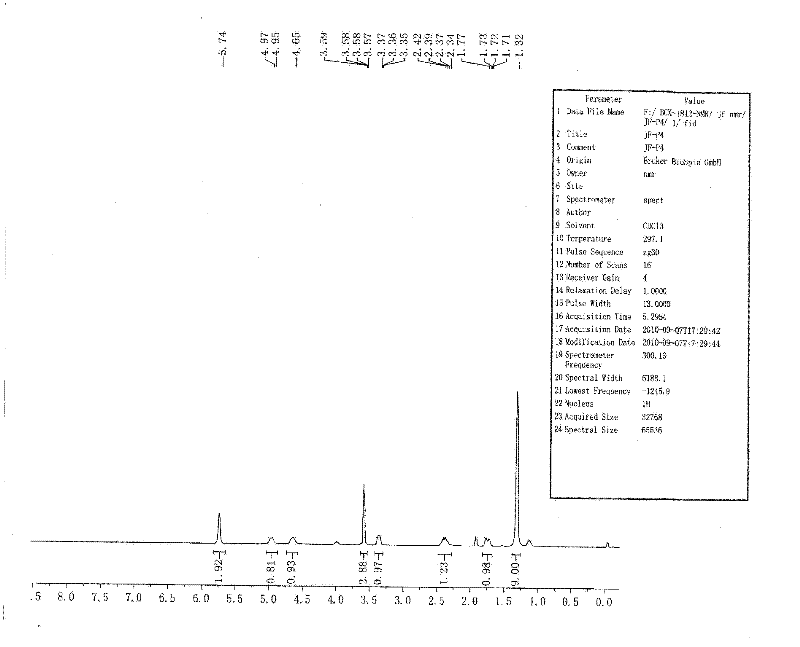
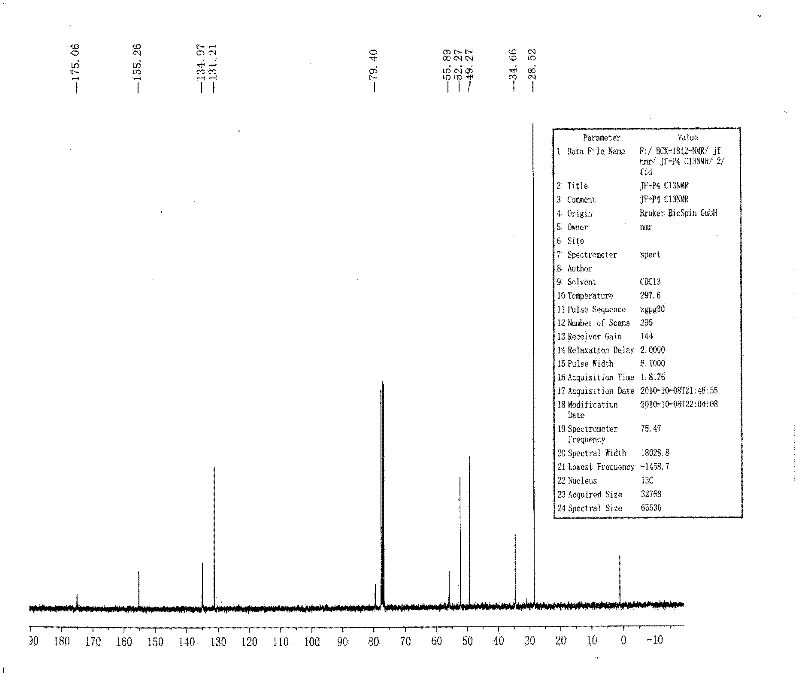
[0065] Below is the preparation embodiment of Peramivir, including following 9 parts:
[0066] 1. Preparation of (1S, 4R)-4-amino-2-cyclopentene-1-carboxylic acid methyl ester hydrochloride
[0067] Dissolve 10g of (1R,4S)-2-azabicyclo[2.2.1]hept-5-en-3-one in 120ml of dry anhydrous methanol, stir and pass in dry hydrogen chloride gas at room temperature for 3h, Hydrogen chloride gas was no longer introduced; the reaction was carried out at 50° C. for 2 h, monitored by TLC. After the reaction was completed, the reaction solution was concentrated to dryness to obtain a black viscous liquid. Add 120ml of ice-free anhydrous ether to the viscous substance, and stir overnight at room temperature. A white solid precipitated, and was filtered with suction, and the filter cake ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com