Method for preparing hydroxyl fasudil compounds
A technology for fasudil hydrochloride and a compound, applied in the field of drug synthesis, can solve the problems of process limitation, inability to realize large-scale production, labor-intensive and time-consuming, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
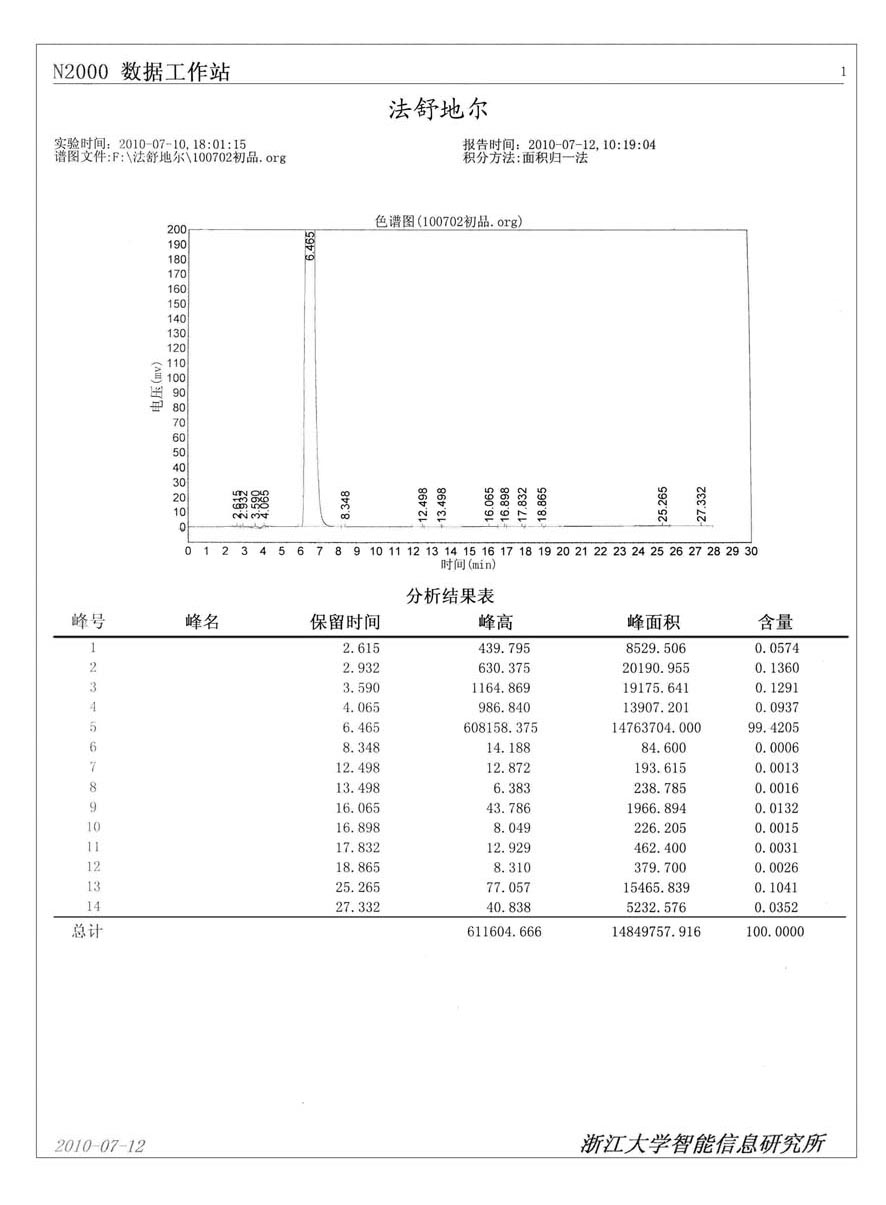
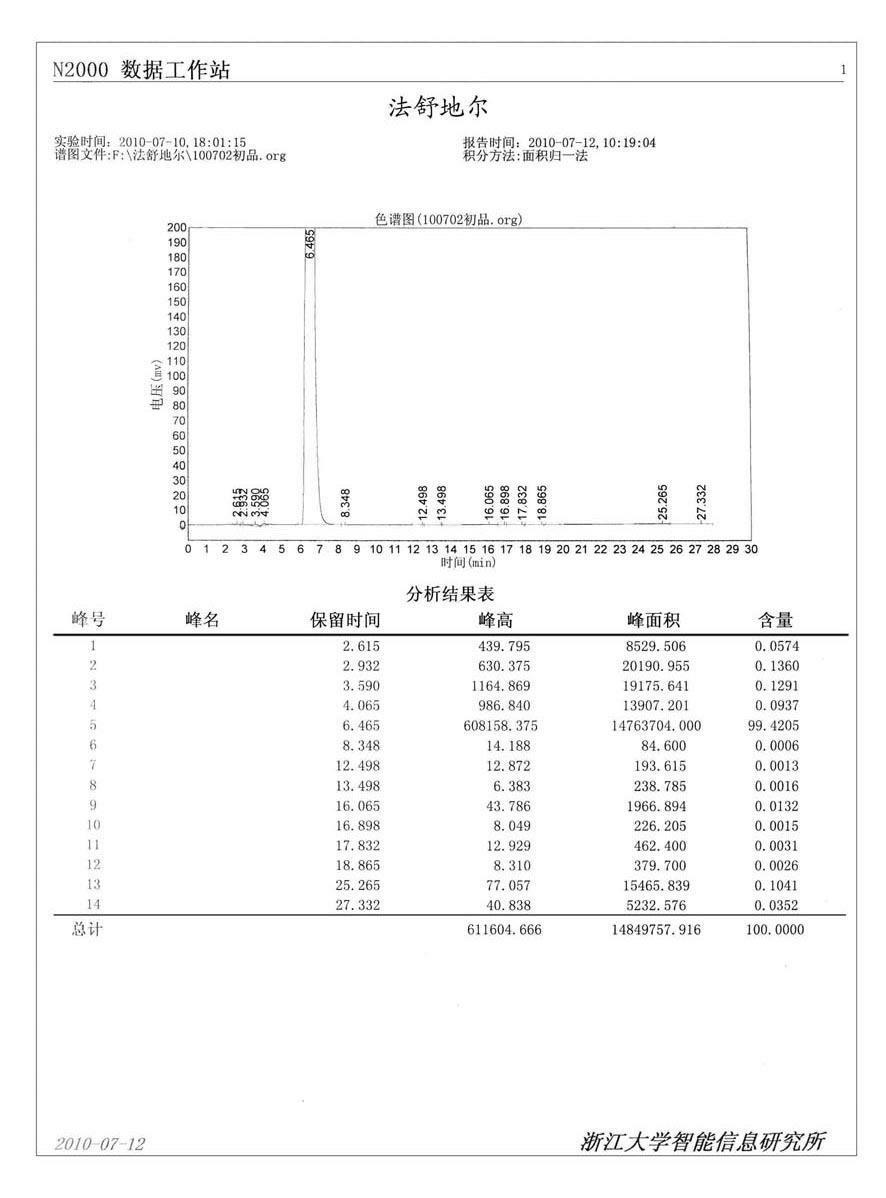
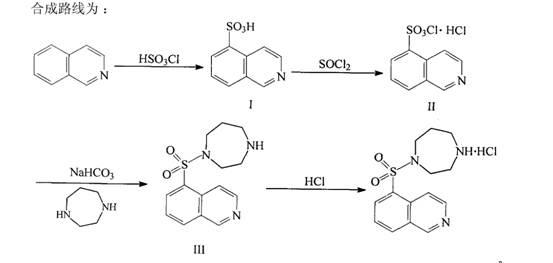
[0060] Add 100g (0.38mol) of 5-isoquinolinesulfonyl chloride hydrochloride, 50g (0.60mol) of sodium bicarbonate solid, and 700 ml of dichloromethane into a 2L three-necked flask, cool to -12°C, and Add 600ml of water dropwise at ~-12°C, after dropping, continue to stir for 15 minutes, let stand to separate layers, separate the dichloromethane solution layer containing isoquinolinesulfonyl chloride, and set aside;
[0061] Add 100g (1mol) of homopiperazine to a 2L three-necked flask, add 200ml of dichloromethane, stir mechanically to dissolve into a homogeneous solution, cool to 0°C in ice, and add dropwise isoquinolinesulfonyl chloride dichloromethane solution at 0-5°C , After adding, continue to stir for 5 hours, and detect that the reaction is complete.
[0062] Add 2N hydrochloric acid to adjust the pH to 5-6, separate to remove dichloromethane, go to recovery, add 10% sodium hydroxide solution dropwise to the water layer to adjust the pH to 9-10, add 500ml of dichlorometha...
Embodiment 2
[0068] Add 100 g (0.38 mol) of 5-isoquinolinesulfonyl chloride hydrochloride, 60 g (0.43 mol) of potassium carbonate solid, and 700 ml of dichloromethane into a 2L three-necked flask, add 700 ml of dichloromethane, and cool to -12 ℃, add 500ml of water dropwise at -8℃~-12℃, after dropping, continue stirring for 15 minutes, let stand to separate layers, separate the dichloromethane solution layer containing isoquinolinesulfonyl chloride, and set aside;
[0069] Add 100g (1mol) of homopiperazine to a 2L three-necked flask, add 200ml of dichloromethane, stir mechanically to dissolve into a homogeneous solution, cool to 0°C in ice, and add dropwise isoquinolinesulfonyl chloride dichloromethane solution at 0-5°C , After adding, continue to stir for 5 hours, and detect that the reaction is complete.
[0070] Add 2N hydrochloric acid to adjust the pH to 5-6, remove dichloromethane for recovery, add 10% sodium carbonate solution dropwise to the water layer to adjust the pH to 9-10, ad...
Embodiment 3
[0073] Add 100g (0.38mol) of 5-isoquinolinesulfonyl chloride hydrochloride, 80g (0.75mol) of sodium carbonate solid, and 700 ml of dichloromethane into a 2L three-necked flask, cool to -12°C, and Add 630ml of water dropwise at -12°C, after dropping, continue to stir for 15 minutes, let stand to separate layers, separate the dichloromethane solution layer containing isoquinolinesulfonyl chloride, and set aside;
[0074] Add 100g (1mol) of homopiperazine to a 2L three-necked flask, add 200ml of dichloromethane, stir mechanically to dissolve into a homogeneous solution, cool to 0°C in ice, and add dropwise isoquinolinesulfonyl chloride dichloromethane solution at 0-5°C , After adding, continue to stir for 5 hours, and detect that the reaction is complete.
[0075] Add 2N hydrochloric acid to adjust the pH to 5-6, remove dichloromethane for recovery, add 10% potassium hydroxide solution dropwise to the water layer to adjust the pH to 9-10, add 500ml of dichloromethane for extracti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com