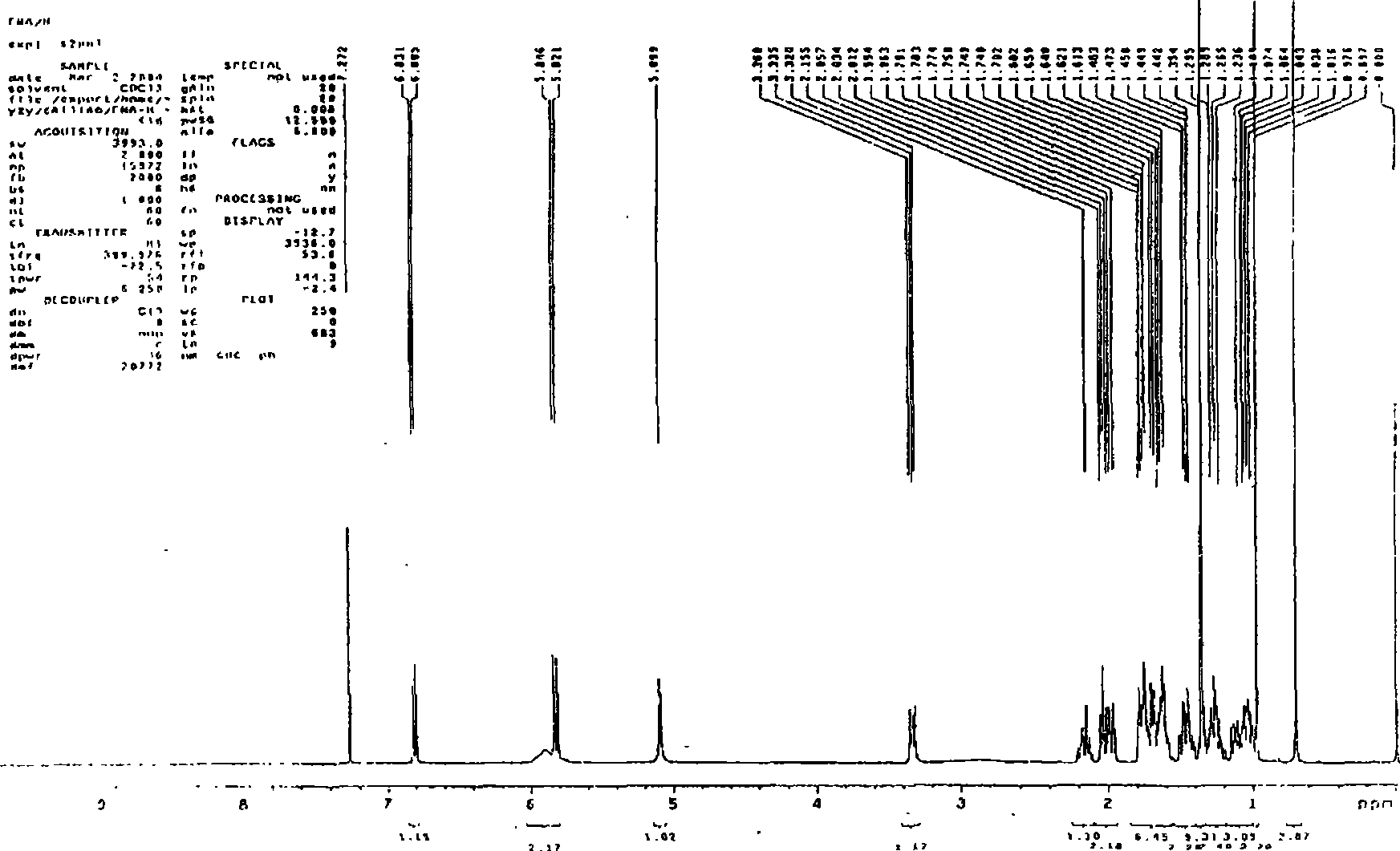
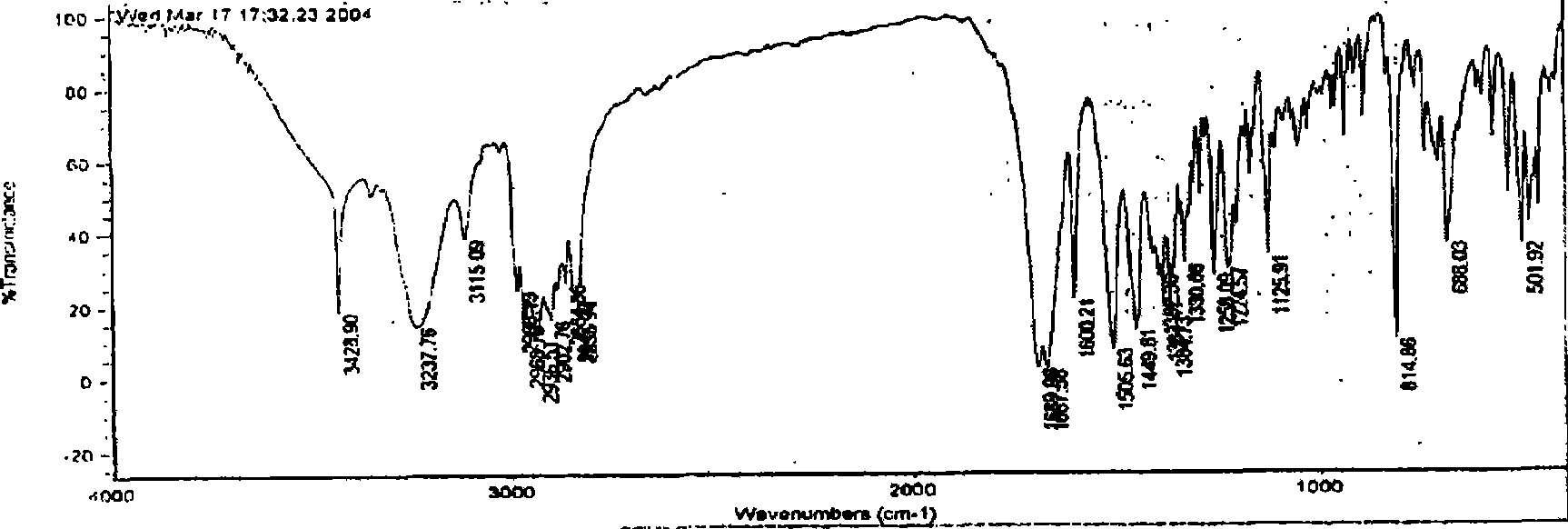
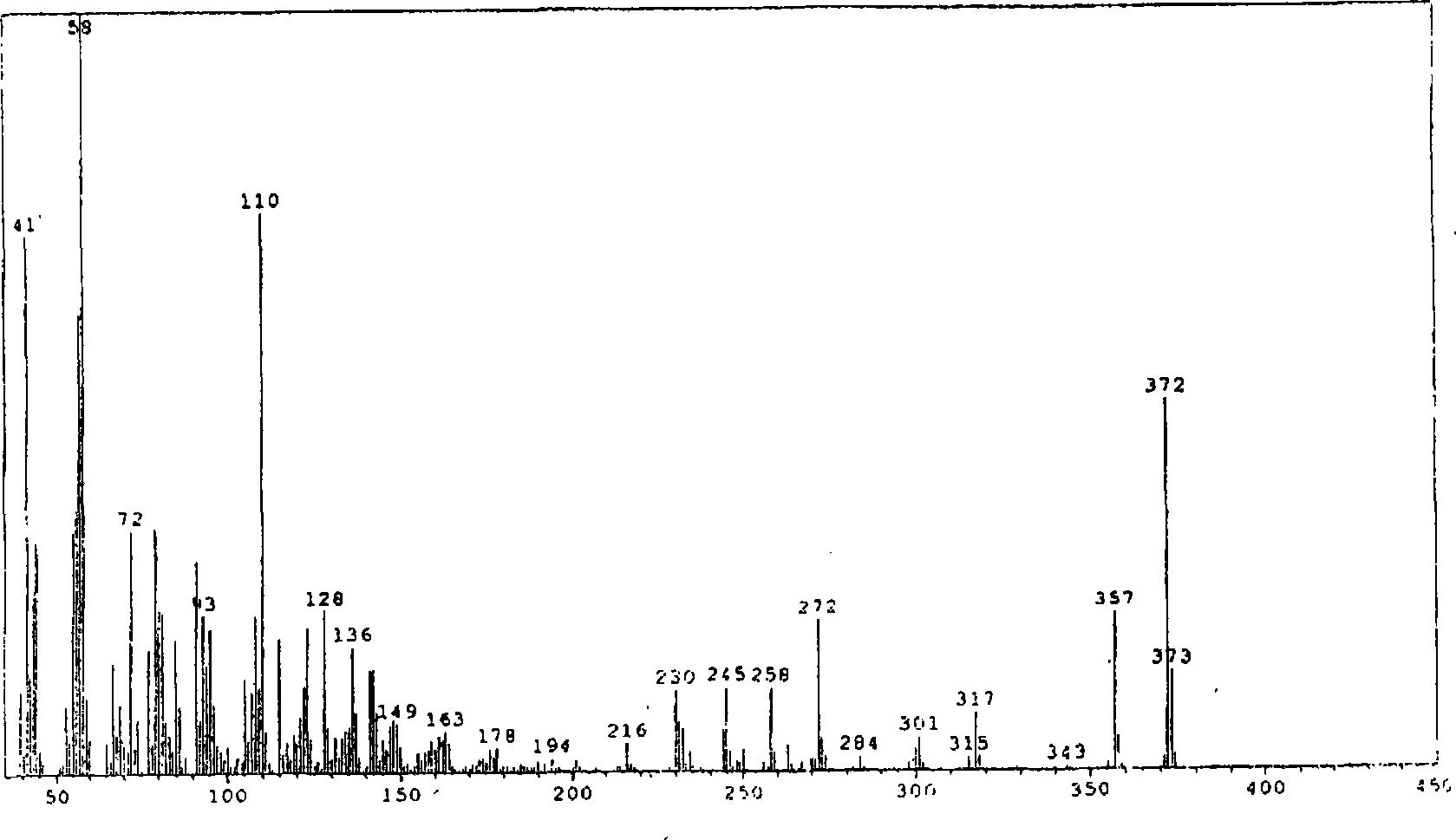
Novel method for synthesizing finasteroid
A technology of finasteride and a synthesis method, applied in the field of drug synthesis, can solve the problems of many reaction steps, high price, complicated operation and the like, and achieves the effects of good product yield, simple operation and prolonged reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Step a: the synthesis of dihydrofinasteride iodide (F9-I)
[0053] In a clean and dry 500ml reaction bottle, 200ml toluene, 20.00g dihydrofinasteride (F 9 ), 25ml triethylamine, blow nitrogen, freeze to 0-5°C, add 13.5ml trimethylchlorosilane, add 20.5g iodine in portions at about 0-5°C, keep warm for 4 hours, monitor by TLC end. After the completion of the reaction, add 100 ml of 20% sodium thiosulfate aqueous solution to quench the reaction; add 100 ml of isopropanol and stir for 2 hours, and separate the liquids; the organic phase is washed once with 100 ml of 20% sodium thiosulfate aqueous solution; phase, back-extract once with a mixture of 20ml isopropanol and 30ml toluene, and discard the water phase; combine the organic phases, wash once with 100ml of 2% aqueous sodium hydroxide solution, and then wash three times with tap water to separate the flocs; Evaporate most of the solvent under reduced pressure, lower to room temperature, slowly add 100ml of petroleum...
Embodiment 2
[0062] Step a: the synthesis of dihydrofinasteride iodide (F9-I)
[0063] In a clean and dry 500ml reaction bottle, 200ml benzene, 20.00g dihydrofinasteride (F 9 ), 30ml of tetramethylethylenediamine, blown nitrogen, freeze to -5°C ~ 0°C, add 16.5ml of iodotrimethylsilane, keep warm at -5°C ~ 0°C, add 25.00g of iodine in stages, after the addition, Continue to incubate the reaction for 6 hours, and monitor the end point by TLC. After the reaction was completed, add 150 ml of a 15% sodium metabisulfite aqueous solution dropwise to quench the reaction; add 100 ml of isopropanol and stir for 2 hours, then separate the liquids; the organic phase was washed once with 150 ml of a 15% sodium metabisulfite aqueous solution; the combined aqueous phase was washed with 20 ml The mixed solution of isopropanol and 30ml of benzene was back extracted once, and the water phase was discarded; the organic phase was combined, washed once with 100ml of 2% aqueous sodium hydroxide solution, and w...
Embodiment 3
[0067] Step a: the synthesis of dihydrofinasteride iodide (F9-I)
[0068] In a clean and dry 500ml reaction bottle, drop 200ml xylene and 20.00g dihydrofinasteride (F 9), 40ml diisopropylethylamine, blow nitrogen, freeze to 0°C~5°C, add 14.5ml trimethylchlorosilane, keep warm at 0°C~5°C, add 18.05g iodine in portions, after the addition is complete, continue the heat preservation reaction 8 hours, TLC monitors the endpoint. After completion of the reaction, add 100ml of 30% sodium sulfite aqueous solution dropwise to quench the reaction; add 100ml of isopropanol and stir for 2 hours, and separate the liquids; the organic phase is washed once with 100ml of 30% sodium sulfite aqueous solution; The mixed solution of alcohol and 30ml xylene was back-extracted once, and the water phase was discarded; the organic phase was combined, washed once with 100ml of 2% aqueous sodium hydroxide solution, and then washed three times with tap water, and the flocs were separated; the organic p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com