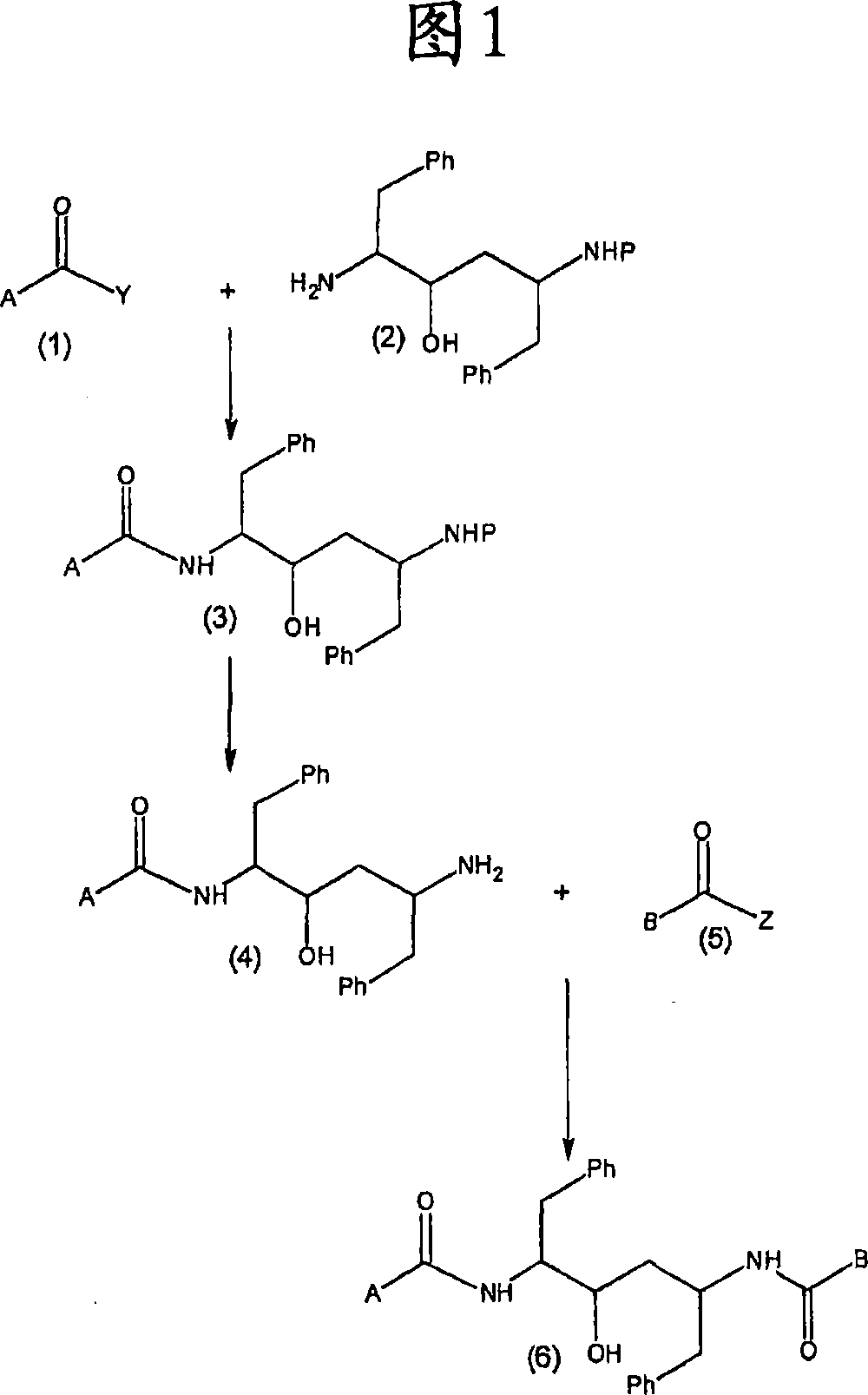
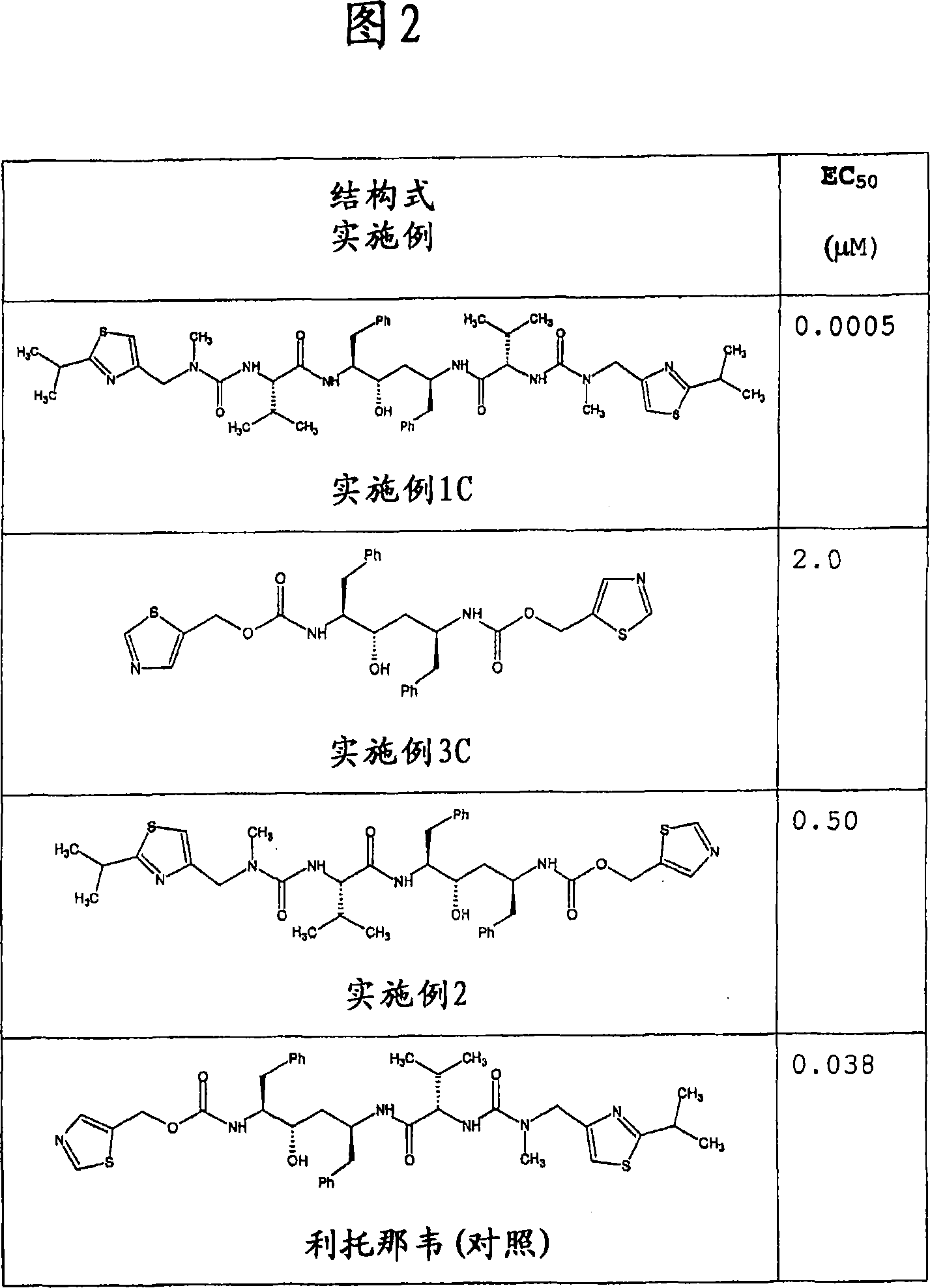
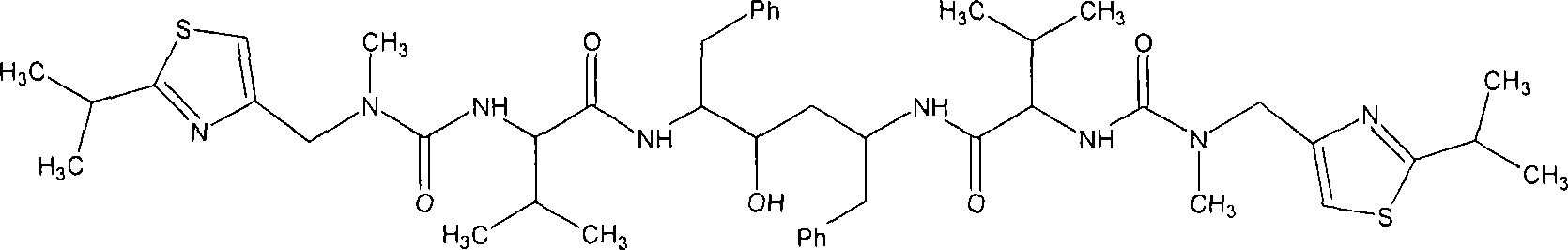
Ritonavir analogous compound useful as retroviral protease inhibitor, preparation of the ritonavir analogous compound and pharmaceutical composition for the ritonavir analogous compound.
A compound and prodrug technology, applied in the field of ritonavir analogs, can solve the problem of lack of antiviral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] compound (2S, 3S, 5S)-2,5-bis-[N-[N-[[N-methyl-N-[(2-isopropyl-4- Thiazolyl)methyl]amino]carbonyl]valyl]amino-1,6-diphenyl-3-hydroxyhexane preparation of
[0063] a. (2S, 3S, 5S)-2-[N-[N-[[N-methyl-N-[(2-isopropyl-4-thiazolyl)methyl Base]amino]carbonyl]valyl]amino]-5-(tert-butoxycarbonylamine)-1,6-diphenyl-3-hydroxyl Hexane
[0064] With stirring system and in N 2 In a 6.0L spherical flask under atmosphere, add (2S, 3S, 5S)-2-amino-3-hydroxyl-5-(tert-butoxycarbonylamine)-1,6-diphenylhexane (100g, 0.26mol ) and chloroform (1.9 L). After the solid had completely dissolved, N-[[N-methyl-N-[(2-isopropyl-4-thiazolyl)methyl]amino]carbonyl]L-valyl hydroxysuccinimide ester (107 g , 0.26mol) in chloroform (630mL). The reaction was monitored by thin layer chromatography and 10% sodium carbonate solution (1.9 L) was added at the end of the reaction. The phases were separated and the organic phase was used directly in the next step without prior purification.
[0065]...
Embodiment 2
[0074] compound (2S, 3S, 5S)-2-[N-[N-[[N-methyl-N-[(2-isopropyl-4-thiazolyl) Methyl]amino]carbonyl]valyl]amino]-5-(N-((5-thiazolyl)methoxycarbonyl)ammonia base)-1,6-diphenyl-3-hydroxyhexane preparation of
[0075] With stirring system and in N 2 In the 4.0L reactor under atmosphere, add THF (2.9L) solution containing the compound obtained in Example 1B and (N-(5-thiazolyl)methyl)-(4-nitrophenyl)carbonate (76g, 0.27mol). The reaction was kept stirring at ambient temperature and the reaction was monitored by TLC. The solvent was removed under reduced pressure, the crude product was dissolved in ethyl acetate (2.0 L), the organic phase was washed with 1N sodium hydroxide solution (8x500 mL) and saturated sodium chloride solution, and dried over sodium sulfate. The product was concentrated under reduced pressure until it reached 1 / 3 of the volume, then hexane was added to crystallize the product. The white solid was dried in a 40°C oven. Yield: 141 g.
[0076] RMN- 1 H...
Embodiment 3
[0079] compound (2S, 3S, 5S)-2,5-bis-(N-((5-thiazolyl)methoxycarbonyl)ammonia base)-1,6-diphenyl-3-hydroxyhexane preparation of
[0080] a. (2S, 3S, 5S)-2-(N-((5-thiazolyl)methoxycarbonyl)amino-5-(tert-butoxycarbonyl Amino)-1,6-diphenyl-3-hydroxyhexane
[0081] With stirring system and in N 2 In a 6.0L spherical flask under atmosphere, add (2S, 3S, 5S)-2-amino-3-hydroxyl-5-(tert-butoxycarbonylamine)-1,6-diphenylhexane (100g, 0.26 mol) in THF (1.9 L) and (N-(5-thiazolyl)methyl)-(4-nitrophenyl) carbonate (76 g, 0.27 mol) in THF (630 mL). The reaction was monitored by thin-layer chromatography, the solvent was removed under reduced pressure, the crude product was dissolved in ethyl acetate (2.0 L), the organic phase was washed with 1N sodium hydroxide solution (8x500 mL) and saturated sodium chloride solution, and dried over sodium sulfate product. The phases were separated and the crude product was concentrated and used directly in the next step without prior purifica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com