Preparation method of ritonavir
A technology for ritonavir and compound, applied in the field of preparation of ritonavir, can solve problems such as unfavorable scale-up production, low yield, high price, etc., and achieves low cost, easy large-scale production, and good application prospect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
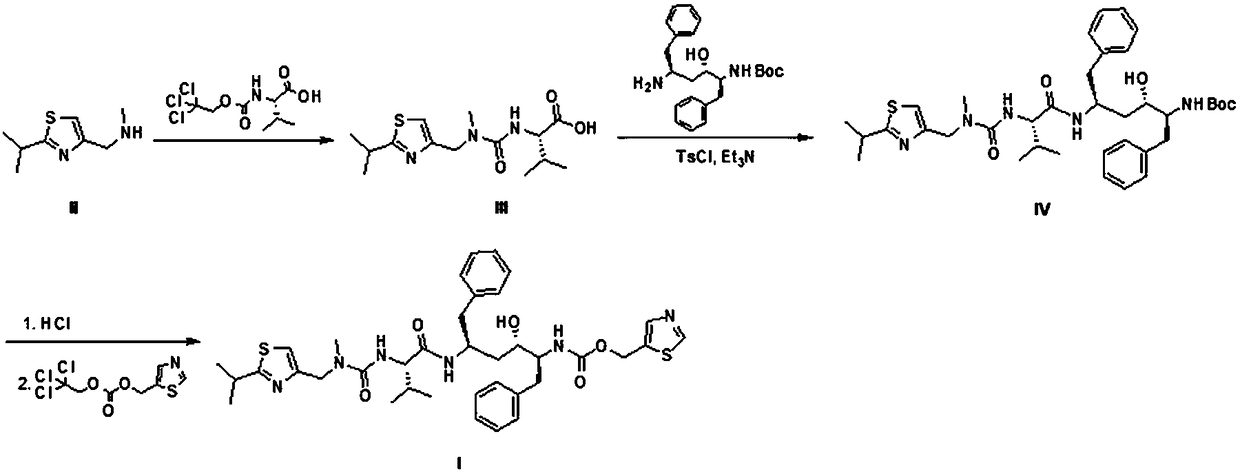
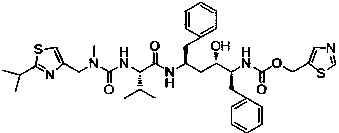
[0043] Such as figure 1 Shown, the preparation method of a kind of ritonavir of the present invention comprises the following steps:
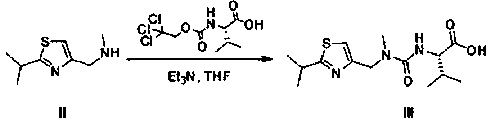
[0044] Step 1, the preparation of compound III, the reaction scheme is as follows:
[0045]
[0046] a. Add (2-isopropylthiazol-4-yl)-nitrogen-methylmethylamine 50.0 g (0.294 mol, 1.0 eq), (S)-3-methyl-2-[(2,2,2 -Trichloroethoxy)formamide]butyric acid 86.0 g (0.294 mol, 1.0 eq), triethylamine 38.6 g (0.382 mol, 1.3 eq) and tetrahydrofuran 250 mL were added to the reaction flask, and the temperature was raised to 50-60°C;
[0047] b, start the reaction until the reaction 6h to the disappearance of raw materials;
[0048] c. After the reaction is completed, cool down to room temperature, pour into 500 mL of 1N hydrochloric acid, and extract with 250 mL of ethyl acetate × 2;
[0049] d. Combine the organic phases, wash with 300 mL of water, and then wash with 300 mL of saturated brine;
[0050] e. Concentrate under reduced pressure to dryne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com