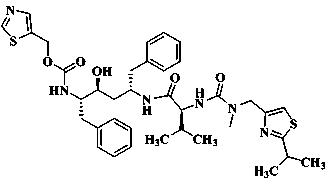
Method for preparing anti-HIV (human immunodeficiency virus) medicine ritonavir
A technology for ritonavir and medicine, which is applied in the field of preparing anti-HIV medicine ritonavir, can solve the problems of being unsuitable for large-scale industrial production, unsatisfactory for industrial production, complicated post-processing process, etc., and achieves easy separation and purification, Less pollution and environment friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (2S,3S,5S)-5-Amino-2-( N Preparation of -((5-thiazolyl)-methoxycarbonyl)amino)-1,6-diphenyl-3-hydroxyhexane
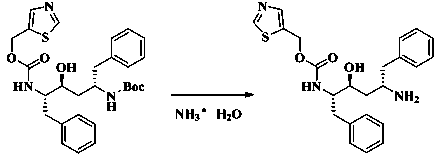
[0018] To a clean reaction flask, add 52.6g (100mmol) of (2S,3S,5S)-5-(tert-butoxycarbonylamino)-2-( N -5-thiazolylmethoxycarbonyl) amino-1,6-diphenyl-3-hydroxyhexane and 150 mL of ethyl acetate, then add 26.2 mL of concentrated hydrochloric acid, gradually produce a slurry, and the resulting slurry Stir at 50°C for 3 h, filter, wash the filter cake twice with 40 mL of ethyl acetate, place the wet filter cake in 130 mL of ethyl acetate, add 9.4% dilute ammonia water dropwise to adjust the pH to 10.5. Take the organic phase and wash it once with 130 mL of 25% sodium chloride aqueous solution, dry it with anhydrous sodium sulfate for 3 hours, filter, and use the filtrate for later use.
Embodiment 2
[0020] Preparation of ritonavir
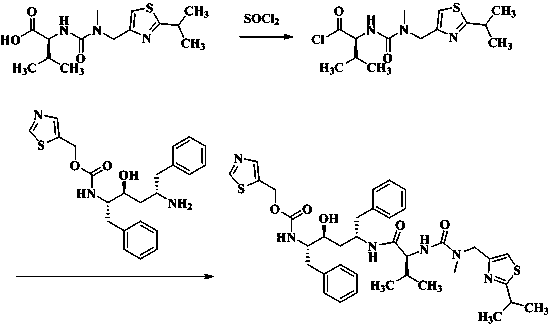
[0021] Will N -[ N -methyl- N -[(2-isopropyl-4-thiazolyl)methyl]aminocarbonyl]-L-valine 62.7g (200.0mmol) was placed in a 500 mL eggplant-shaped bottle, and 200 mL ethyl acetate was added and stirred to make Dissolve it, then add 101.0 g of triethylamine and 25.2 g (212.0 mmol) of thionyl chloride, raise the temperature to 45°C to reflux the ethyl acetate, keep it warm for 3 hours, after the reaction is complete, cool down to room temperature, and filter out the triethylamine salt Salt solid, the filtrate is set aside.
[0022]The filtrate in Example 1 was slowly dropped into the system, stirred overnight, and the reaction was completed. The reaction liquid was washed successively with 10% citric acid, 10% potassium carbonate, 10% sodium chloride and purified water 250 mL×2, the water layer was discarded, and the organic phase was dried by adding anhydrous sodium sulfate for 3 h and then filtered. Then the filtrate was heated to 50°C, and...
Embodiment 3
[0024] (2S,3S,5S)-5-Amino-2-( N Preparation of -((5-thiazolyl)-methoxycarbonyl)amino)-1,6-diphenyl-3-hydroxyhexane
[0025] Into a clean reaction flask, add 36.8g (70mmol) of (2S,3S,5S)-5-(tert-butoxycarbonylamino)-2-( N -5-thiazolylmethoxycarbonyl)amino-1,6-diphenyl-3-hydroxyhexane and 110mL of acetone, then add 18.3 mL of concentrated hydrochloric acid to gradually produce a slurry, and place the resulting slurry in Stir at 50°C for 3 h, filter, wash the filter cake twice with 40 mL of acetone, place the wet filter cake in 100 mL of acetone, add 9.4% dilute ammonia water dropwise to adjust the pH to 10.5, take the organic phase for use Wash once with 120 mL of 25% sodium chloride aqueous solution, dry with anhydrous sodium sulfate for 3 hours, then filter, and the filtrate is set aside.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com