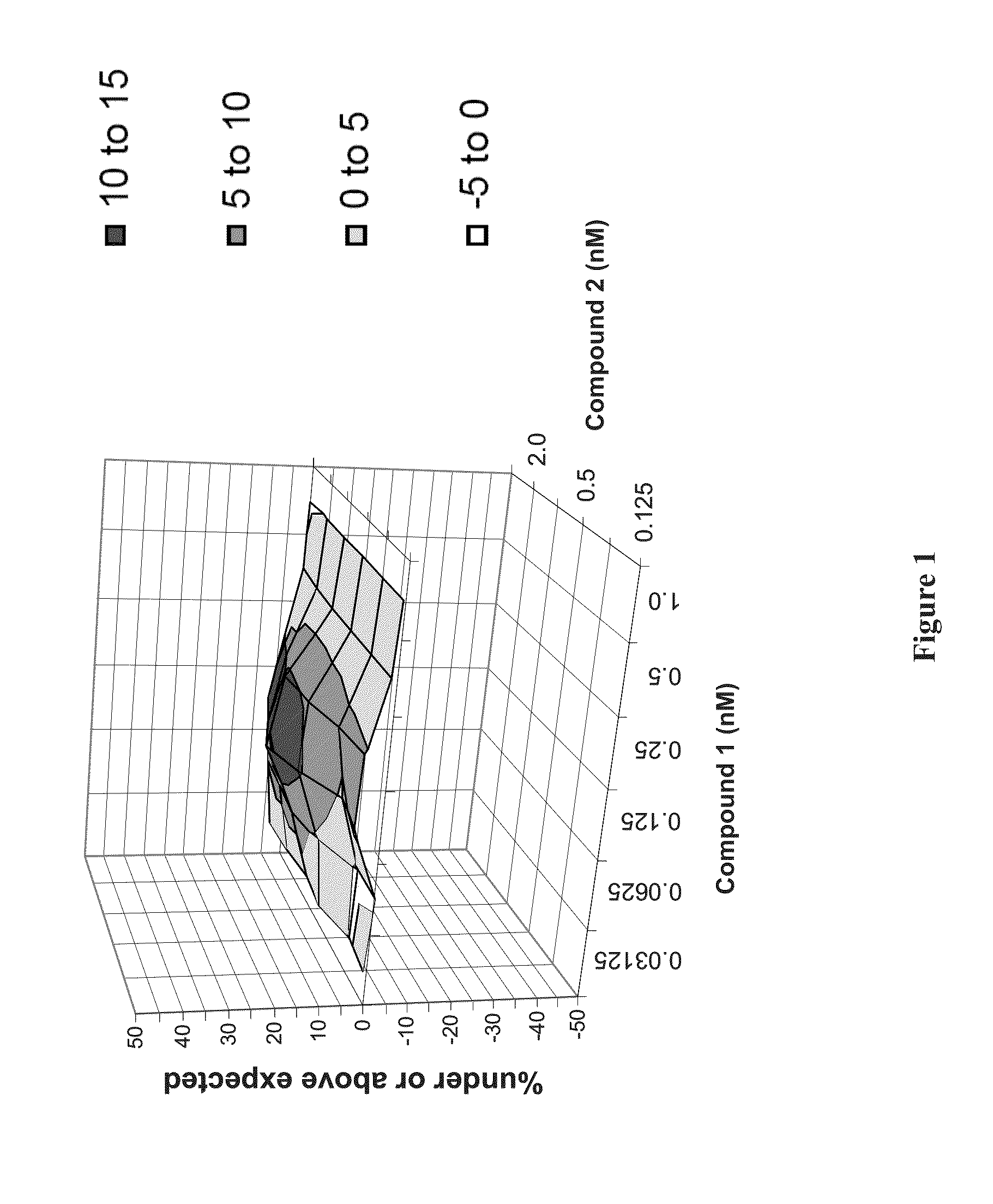
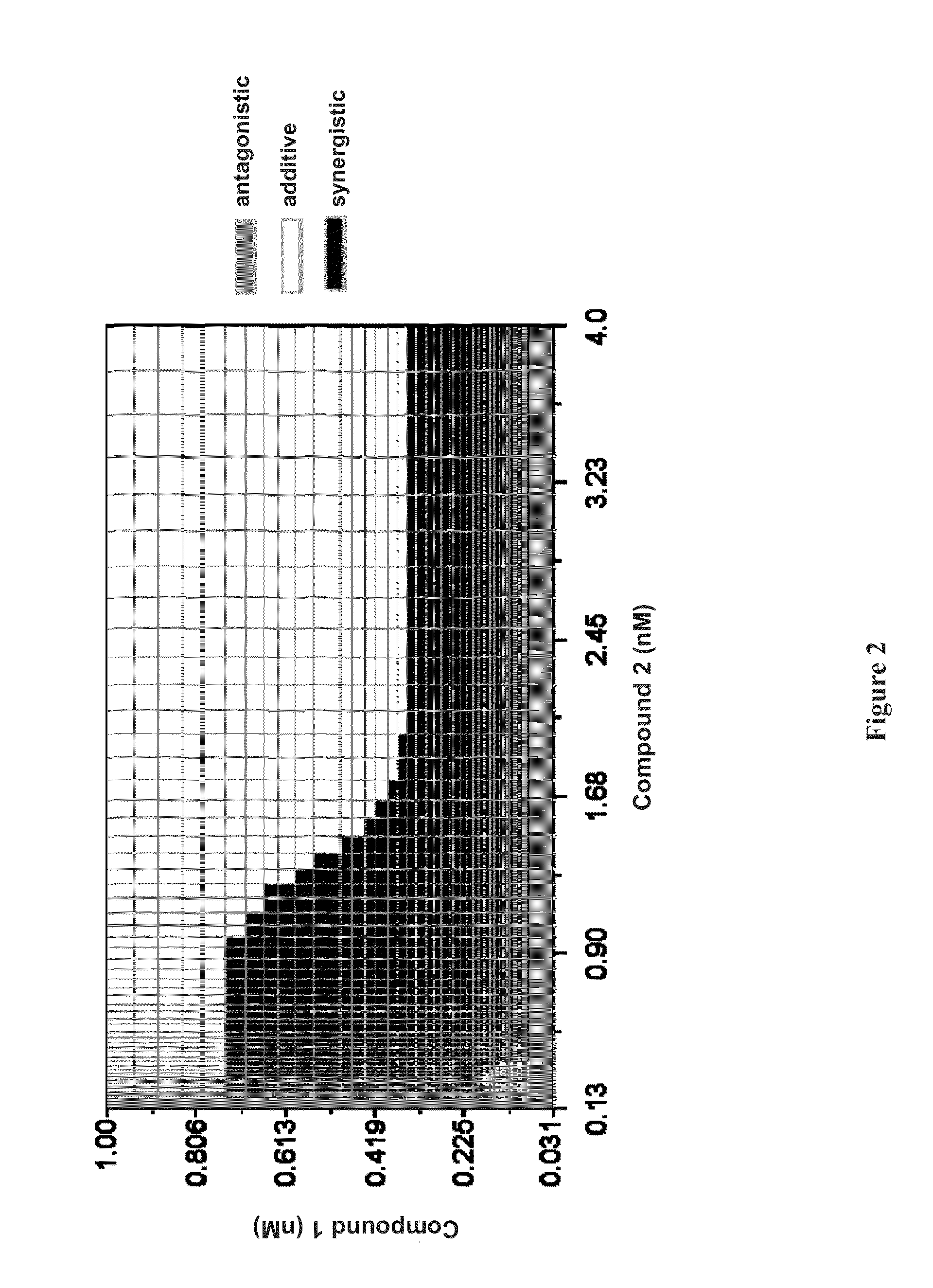
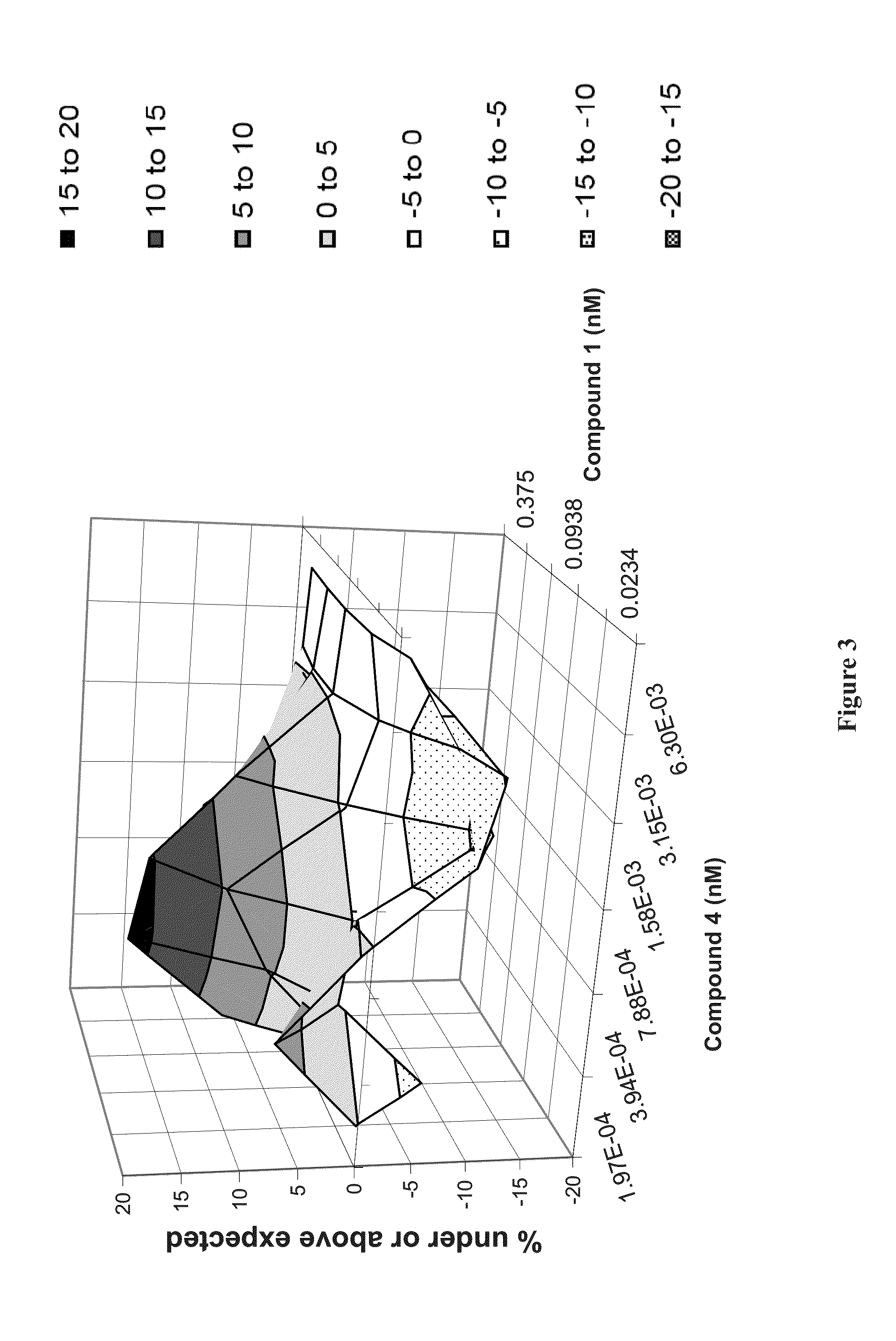
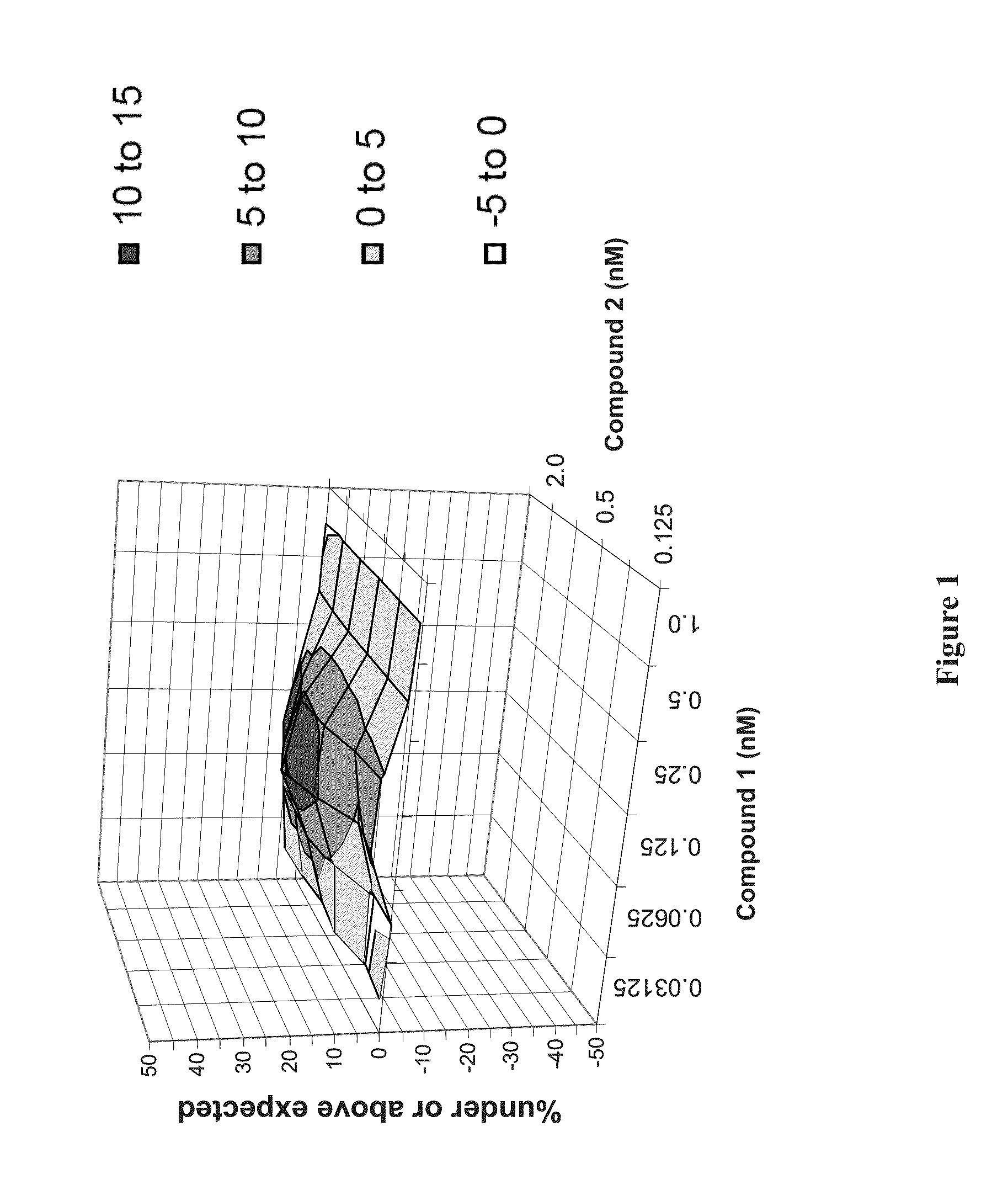
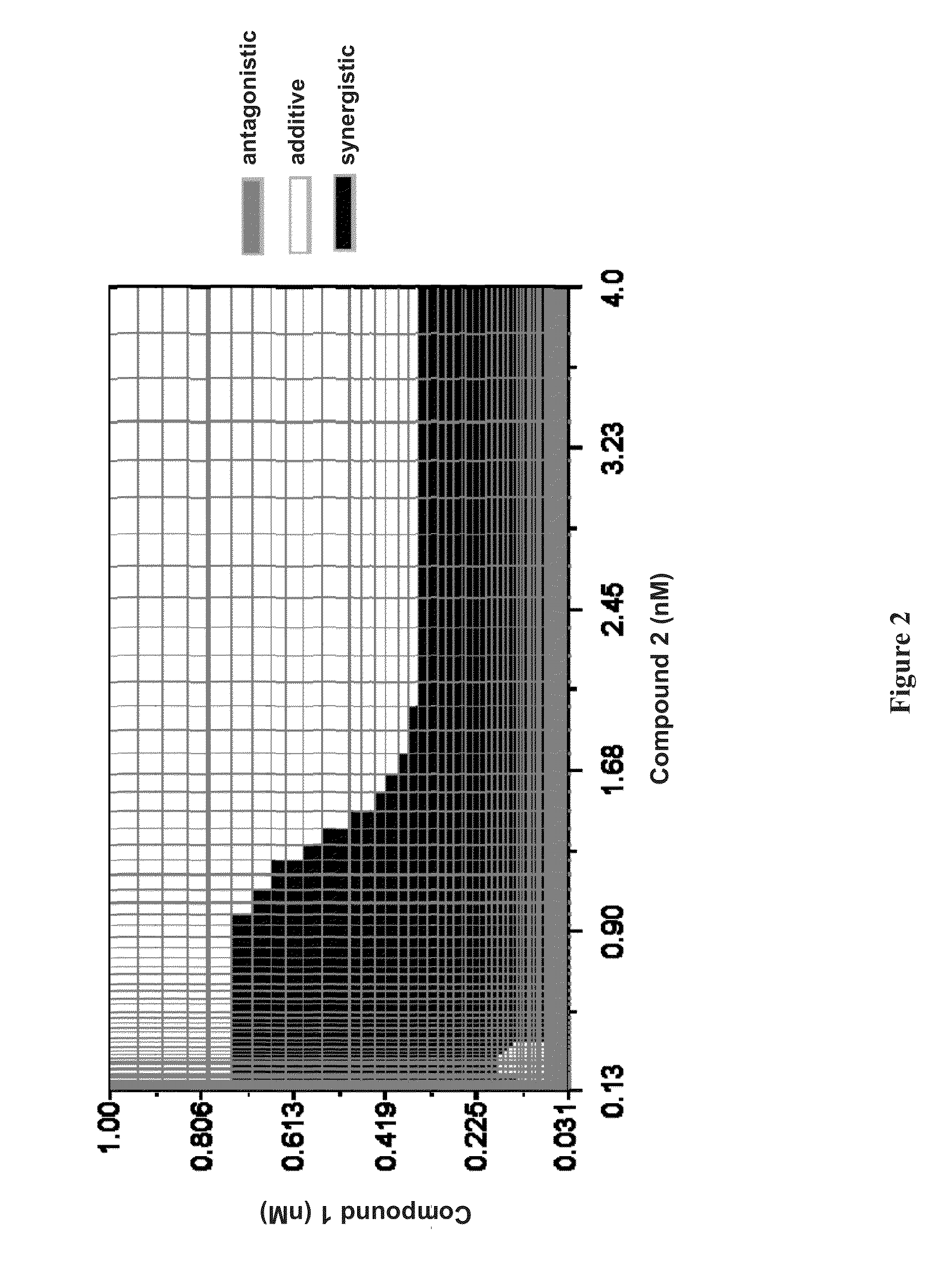
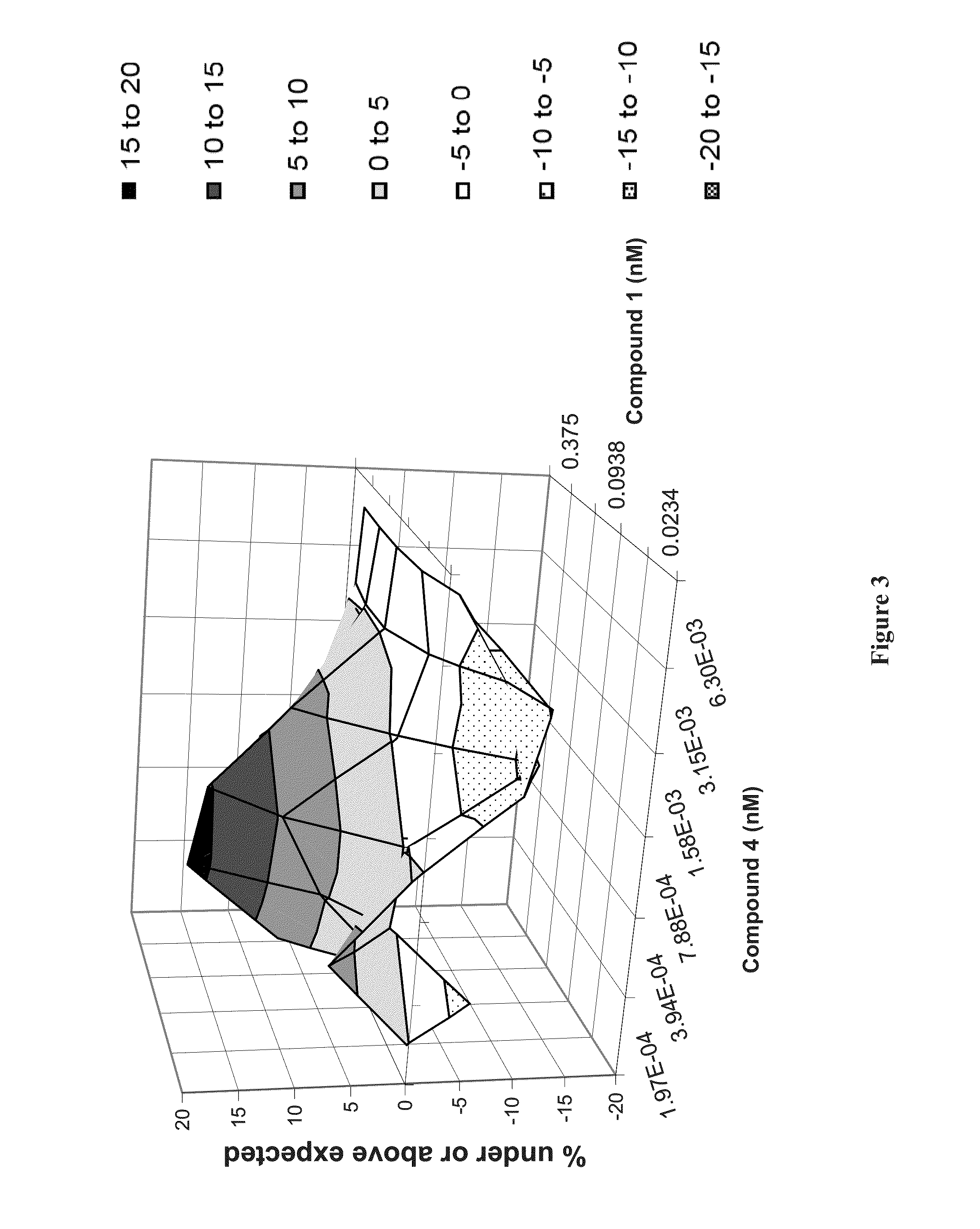
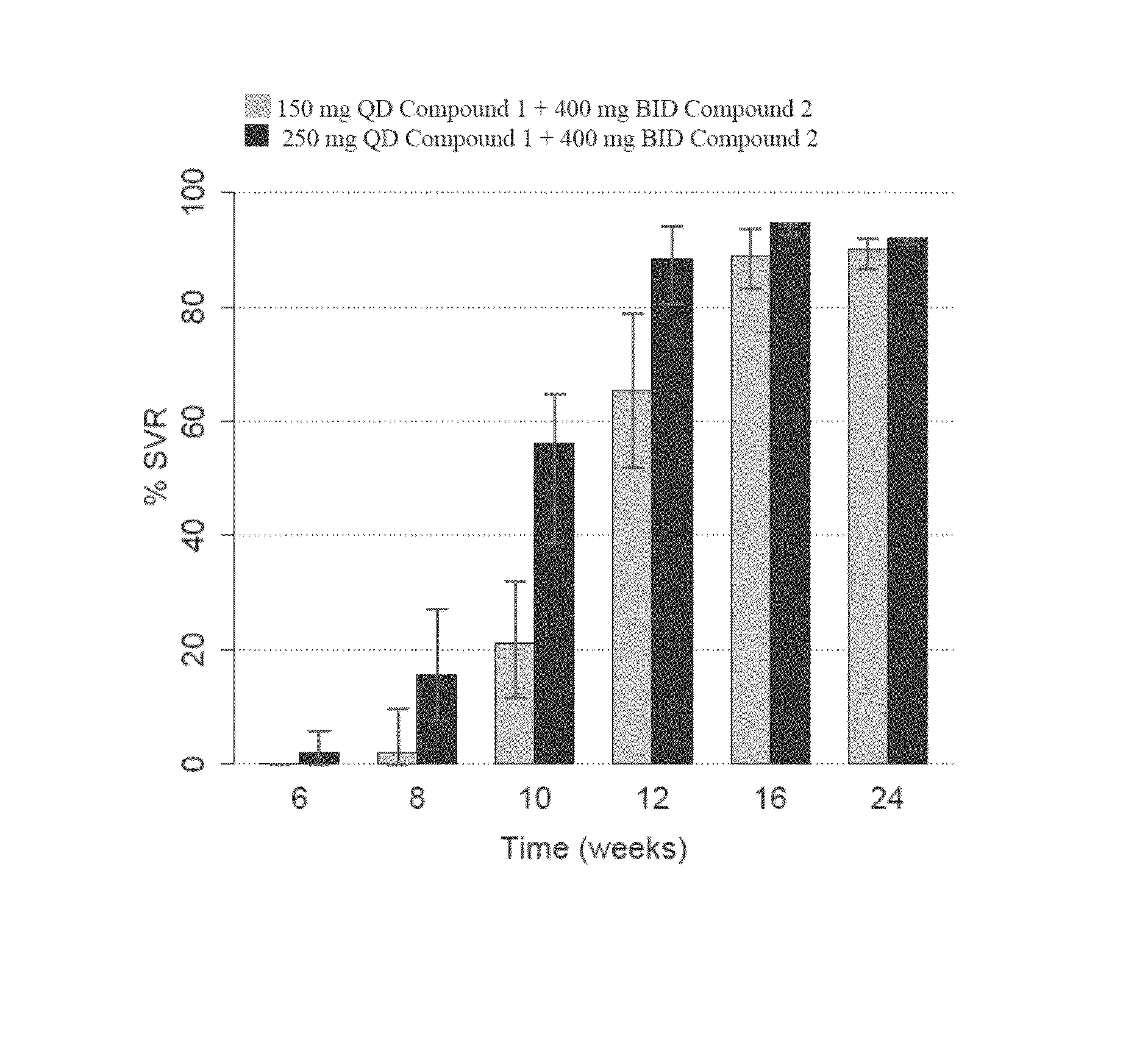
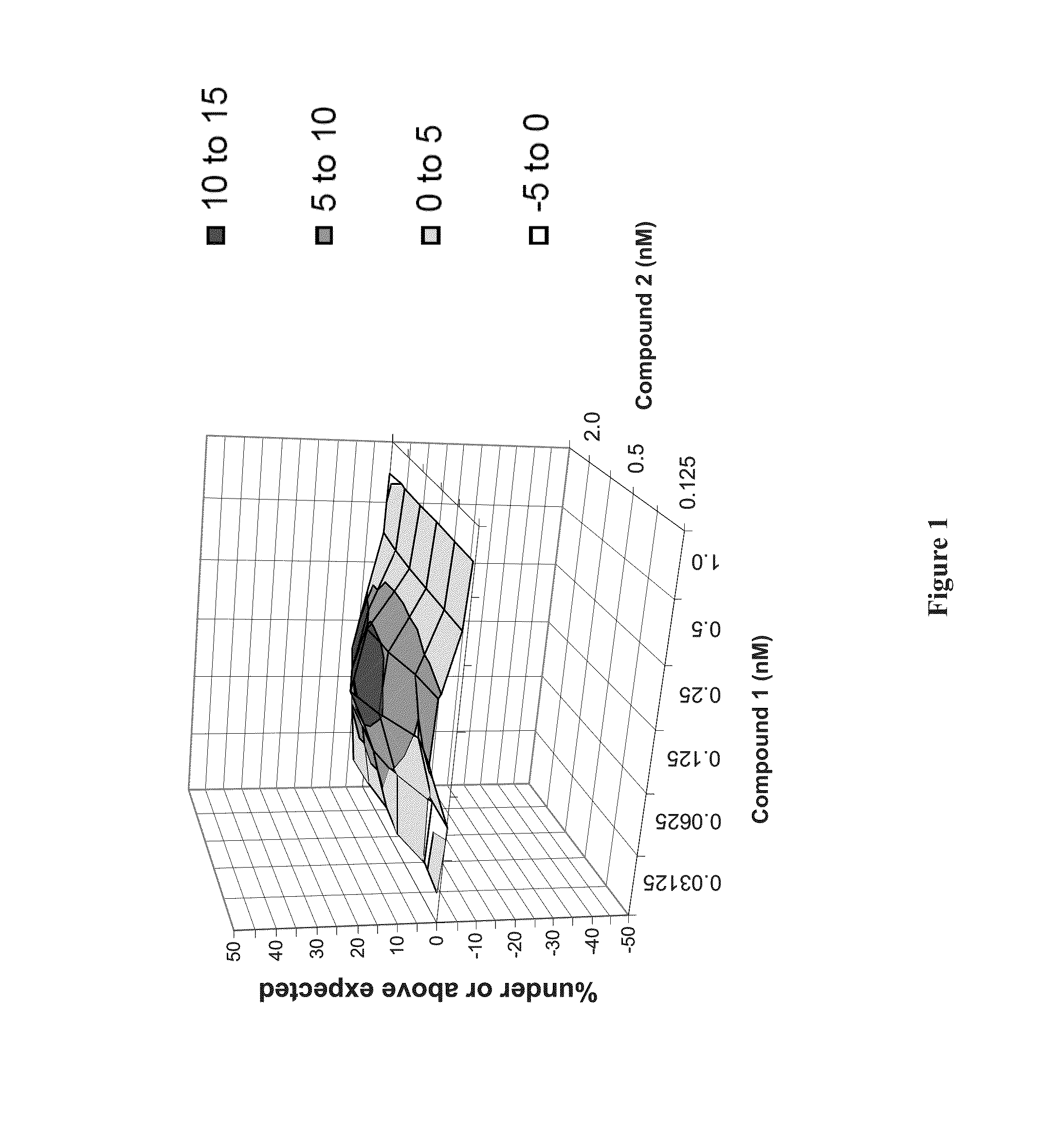
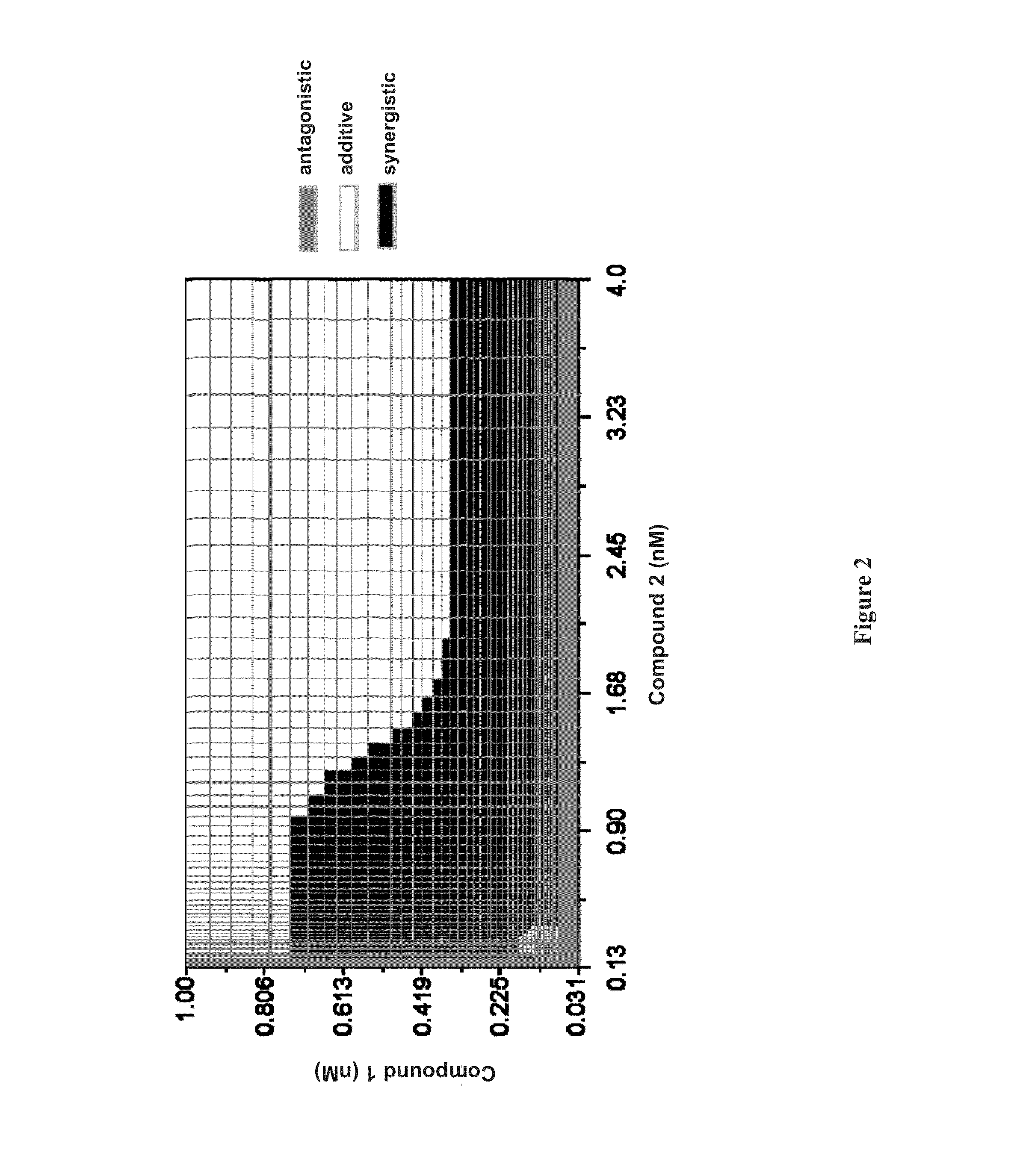
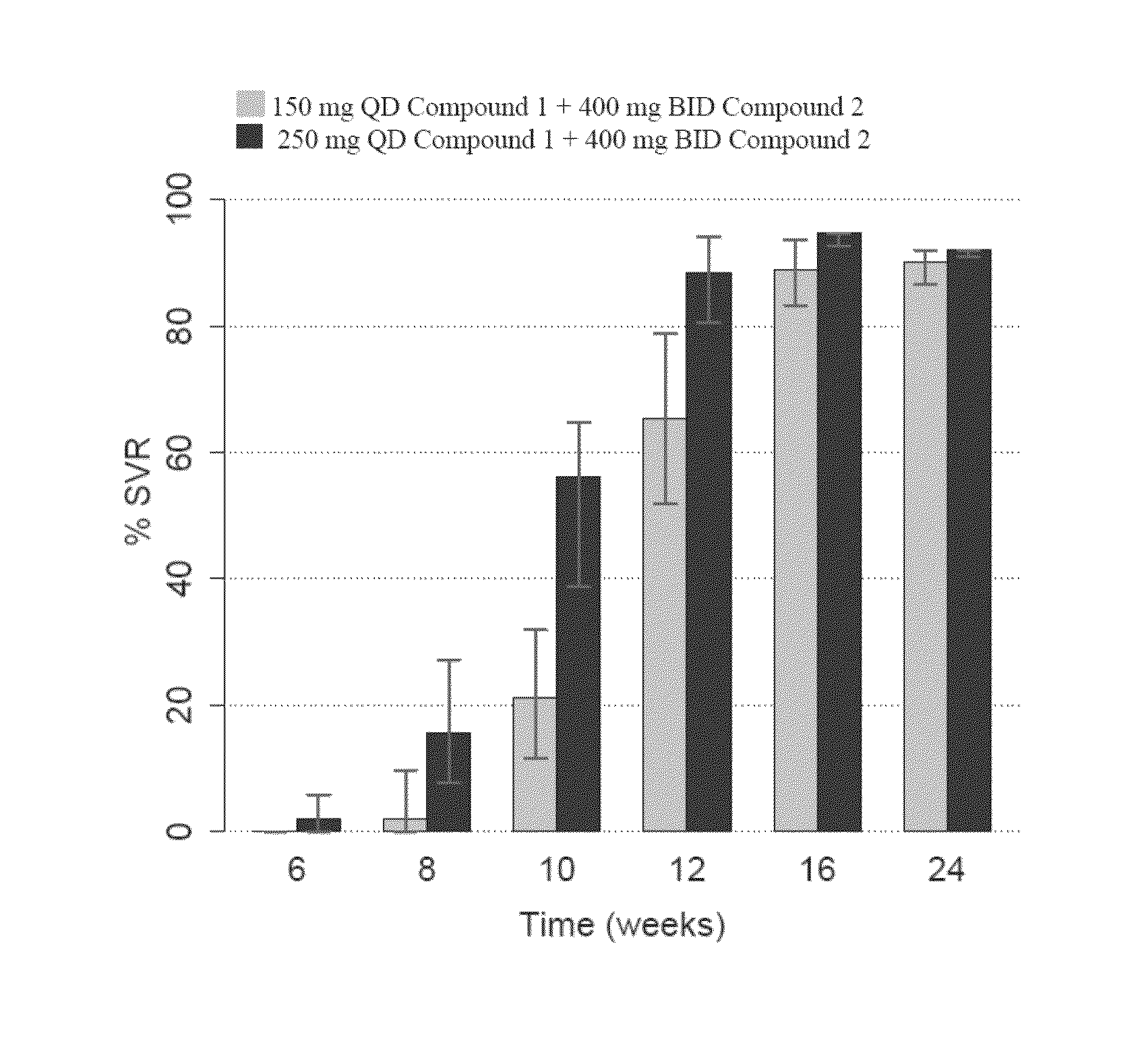
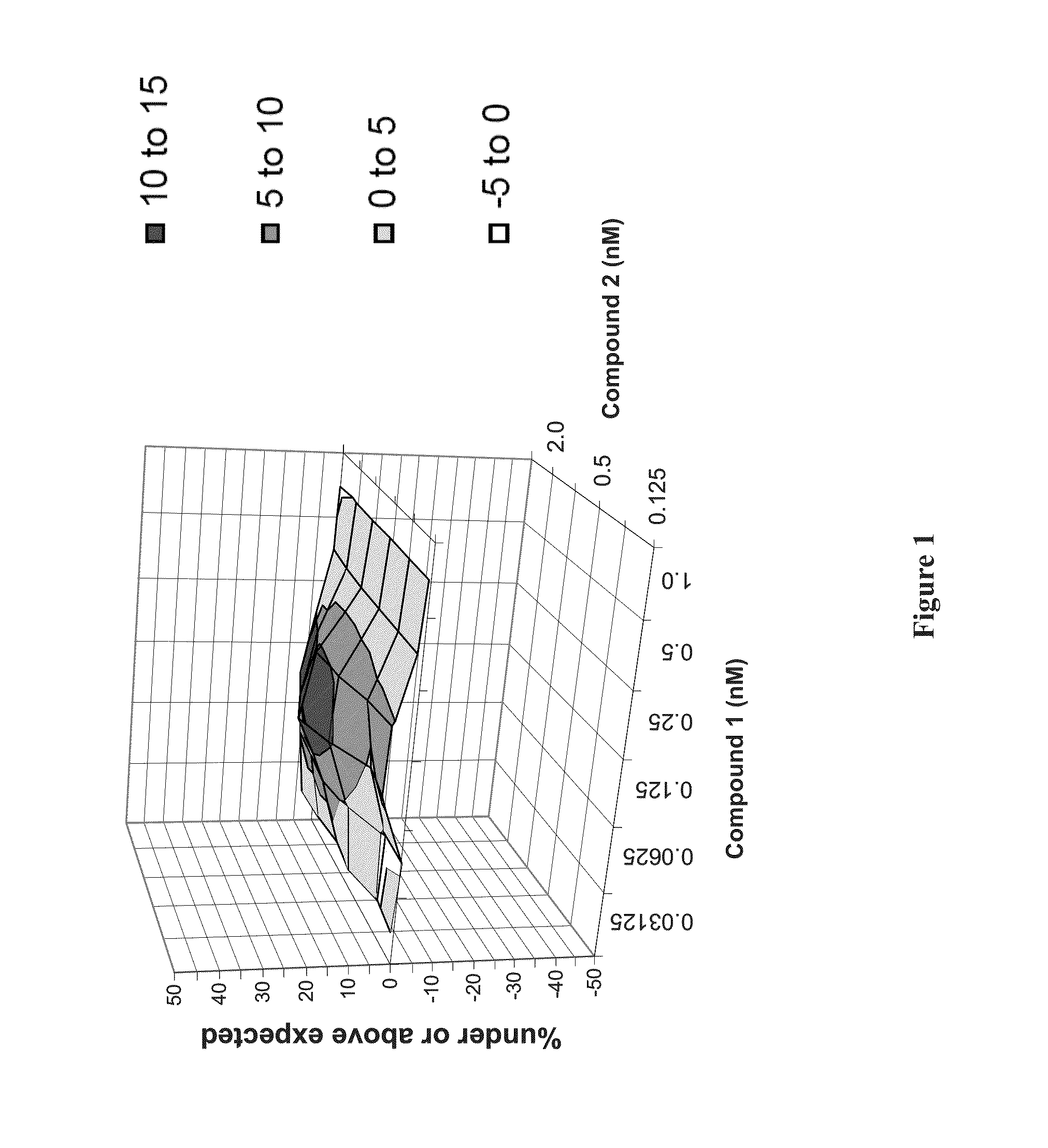
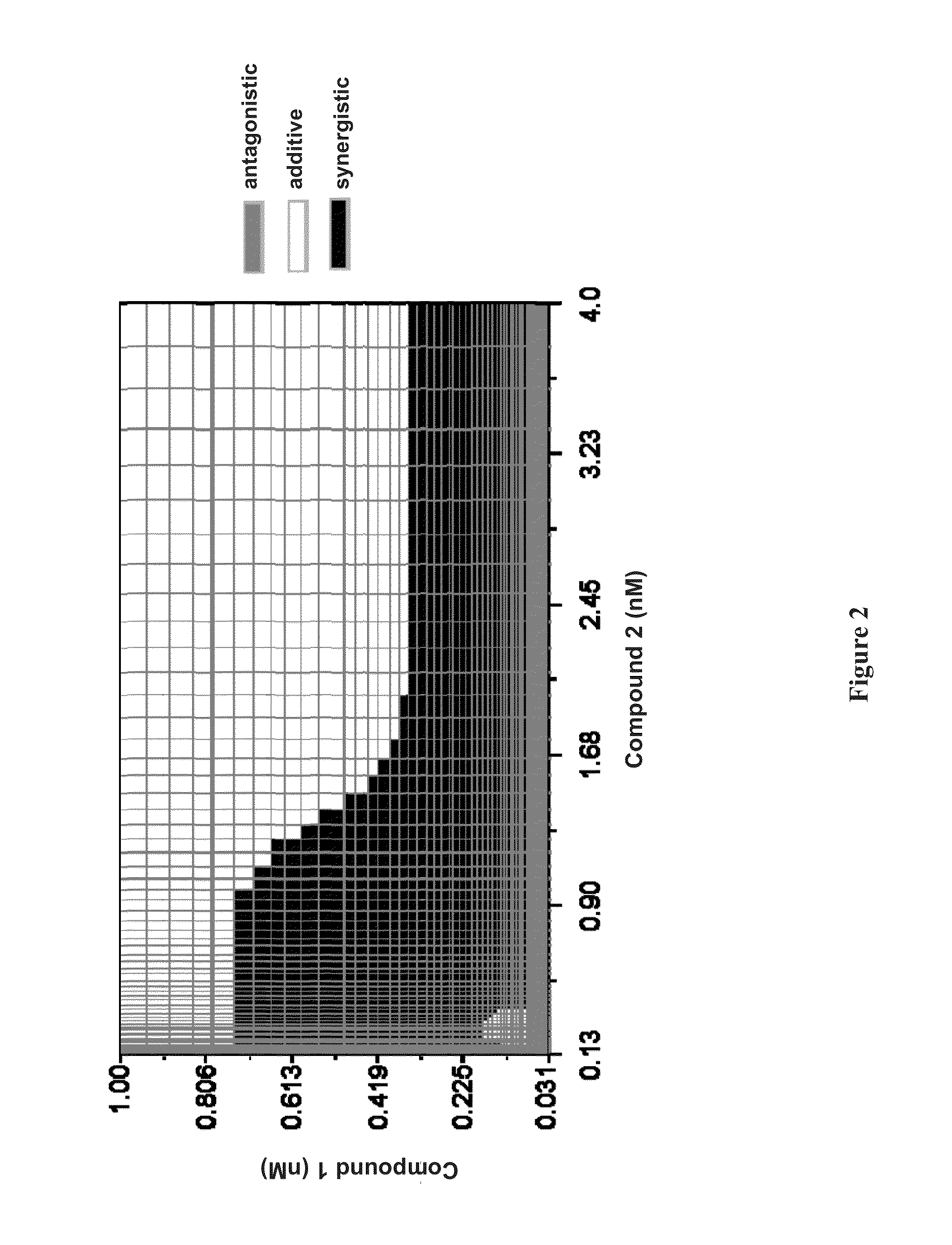
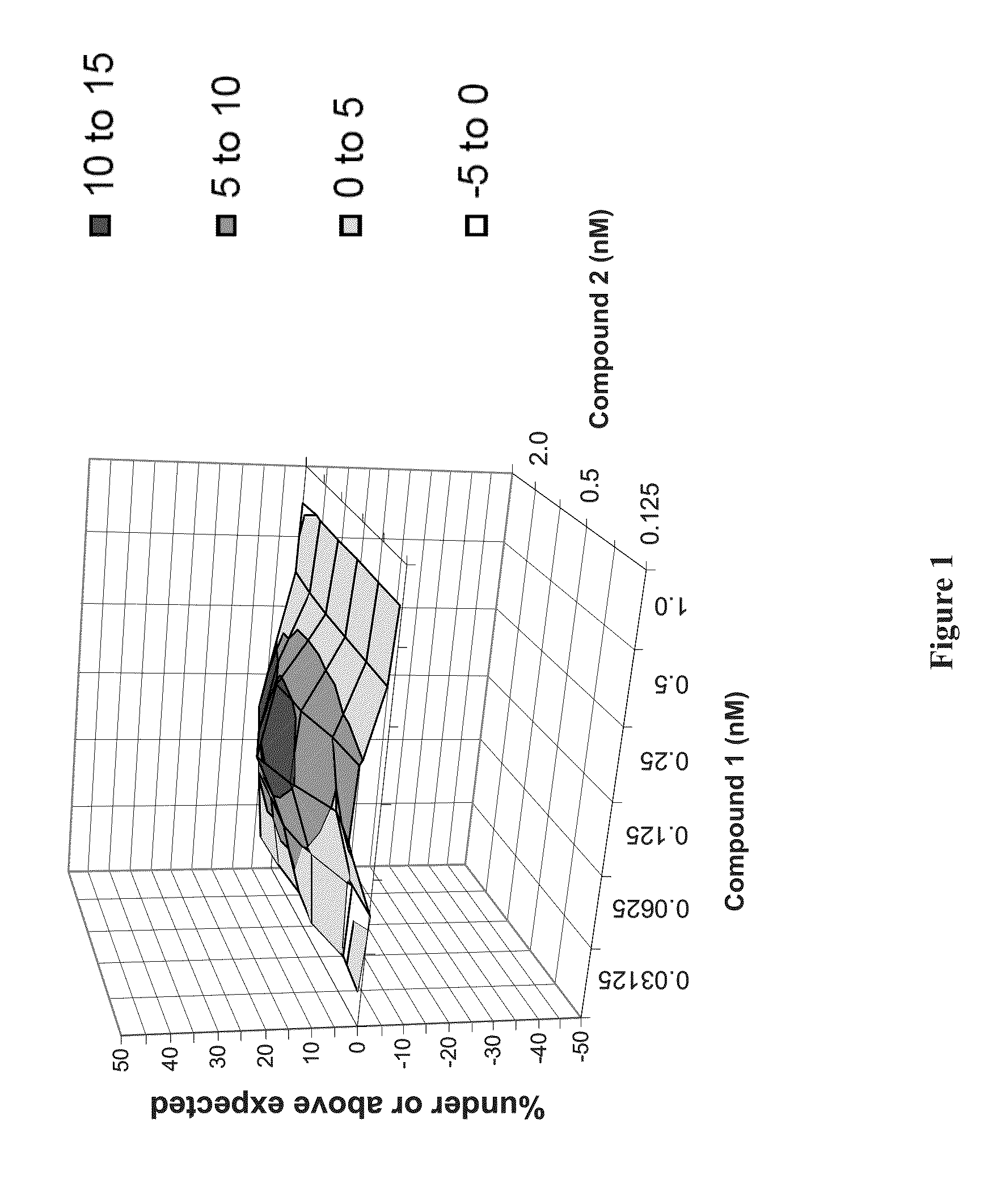
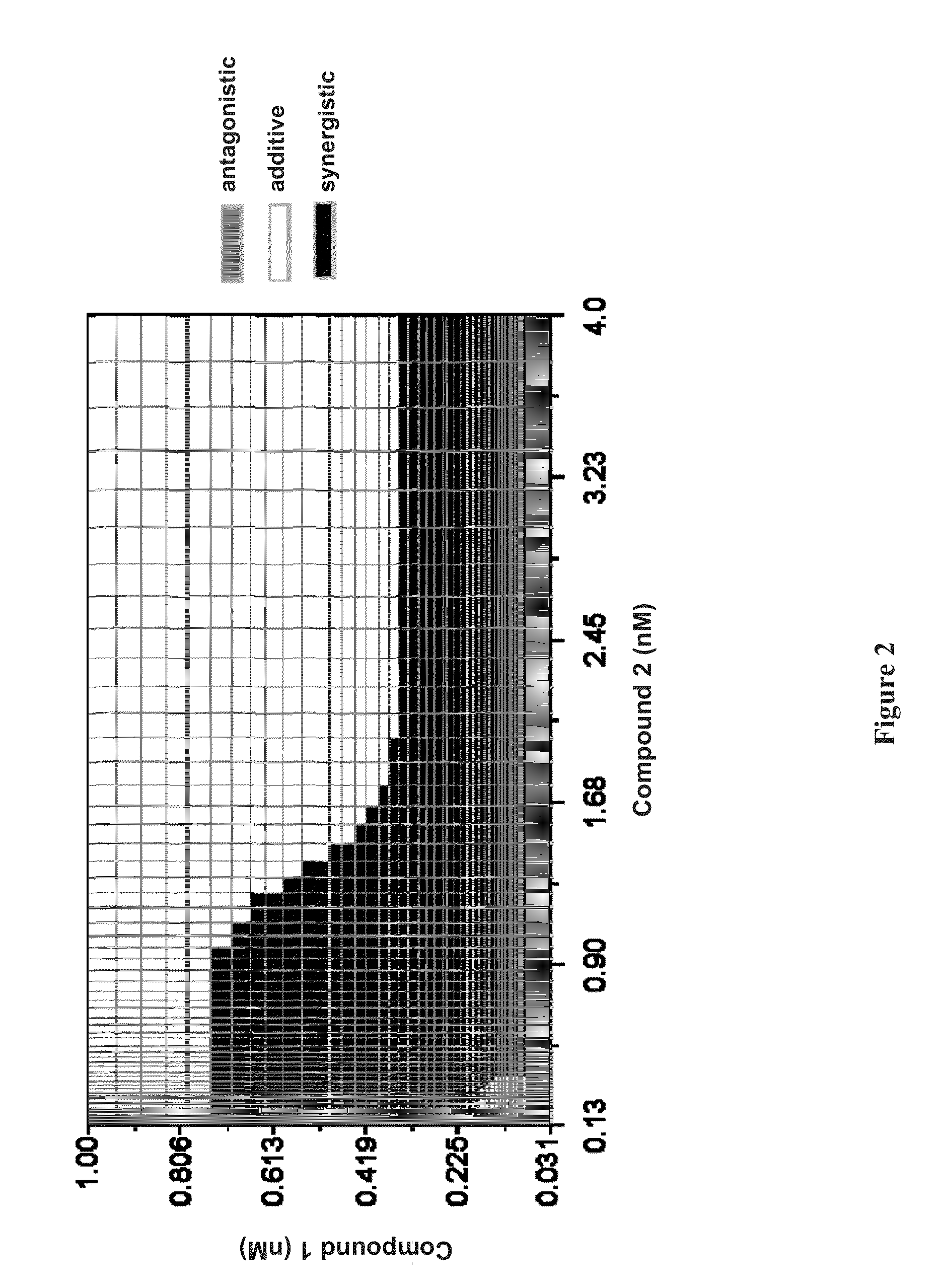
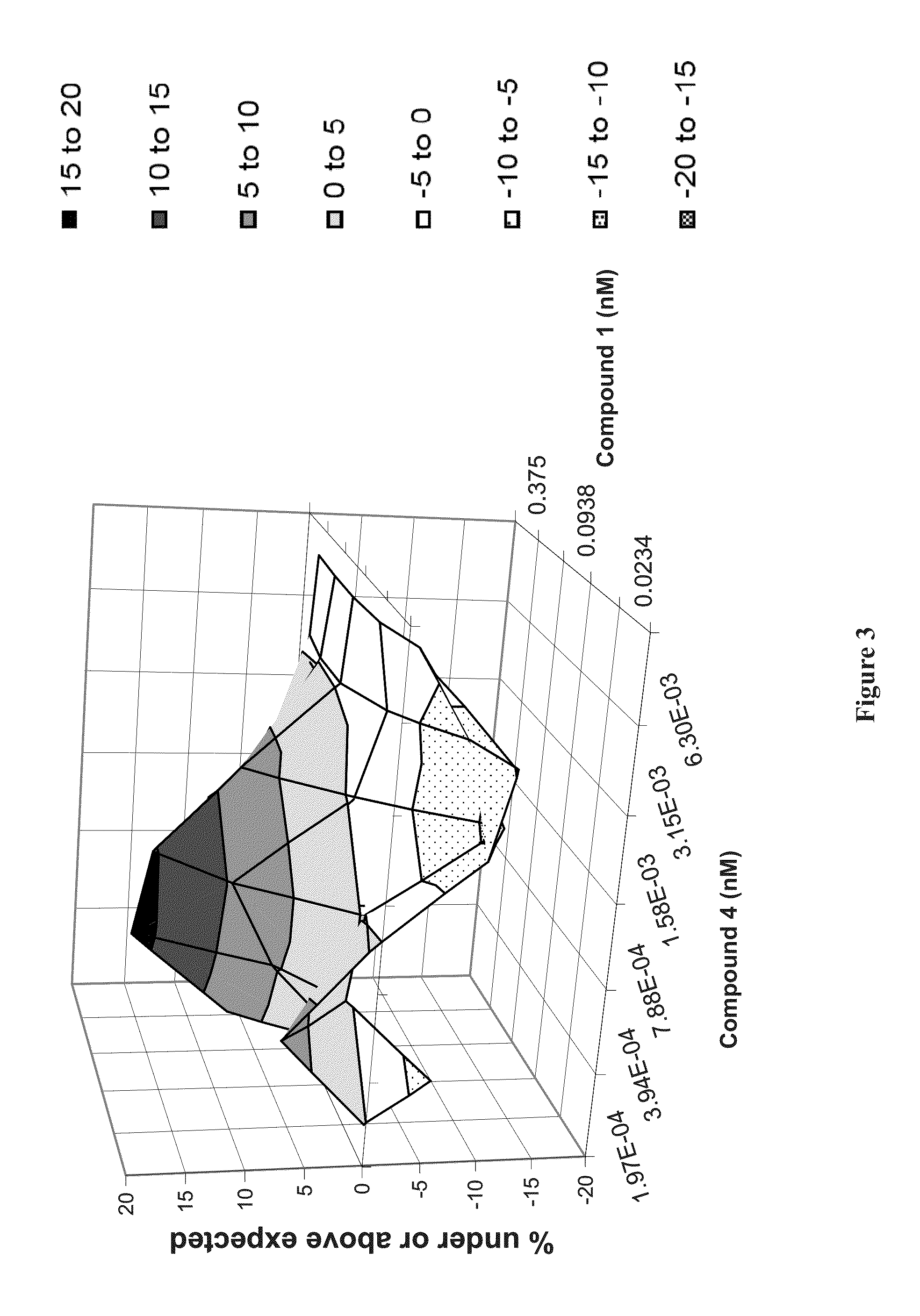
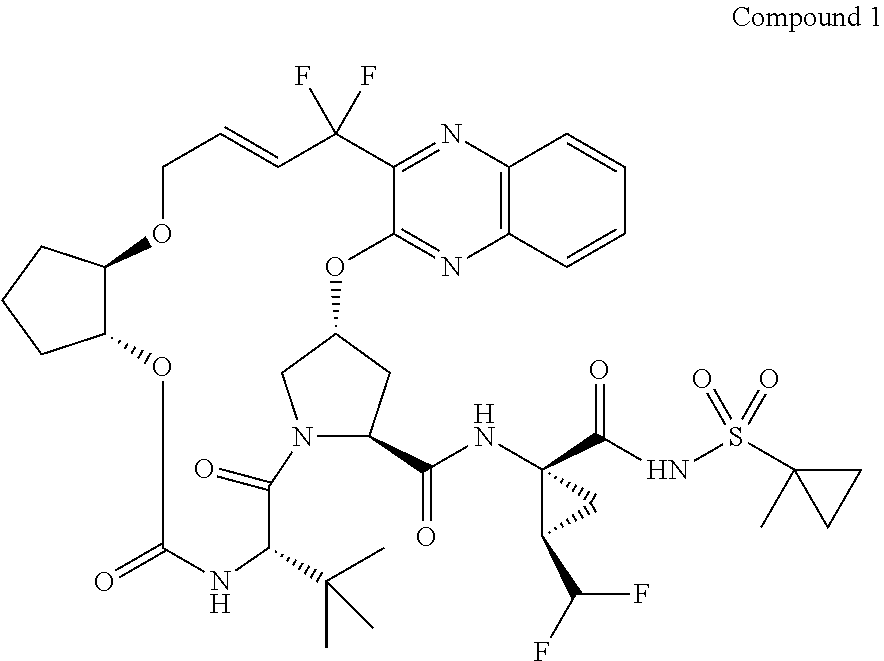
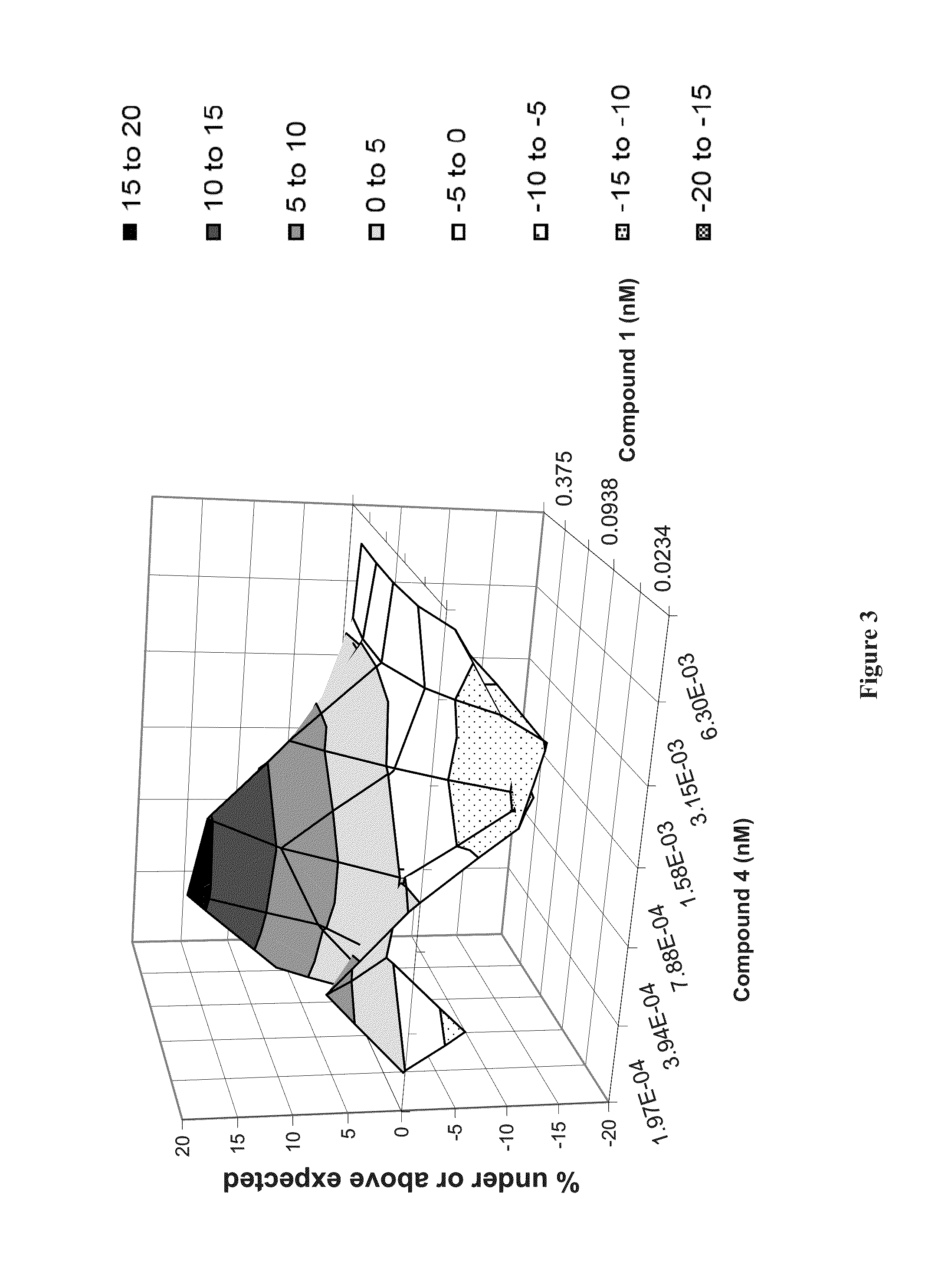
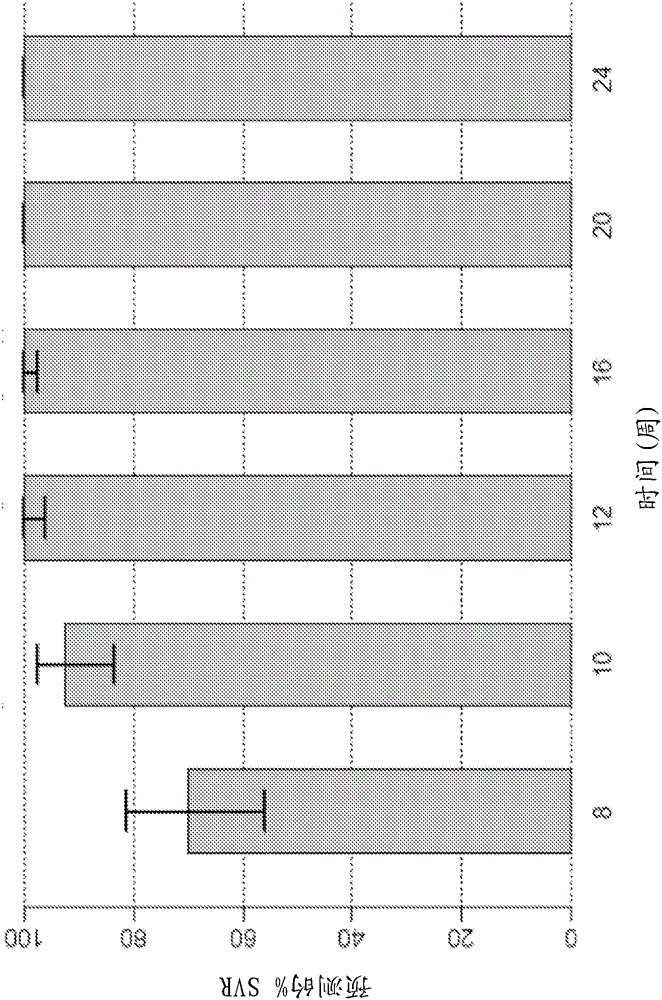
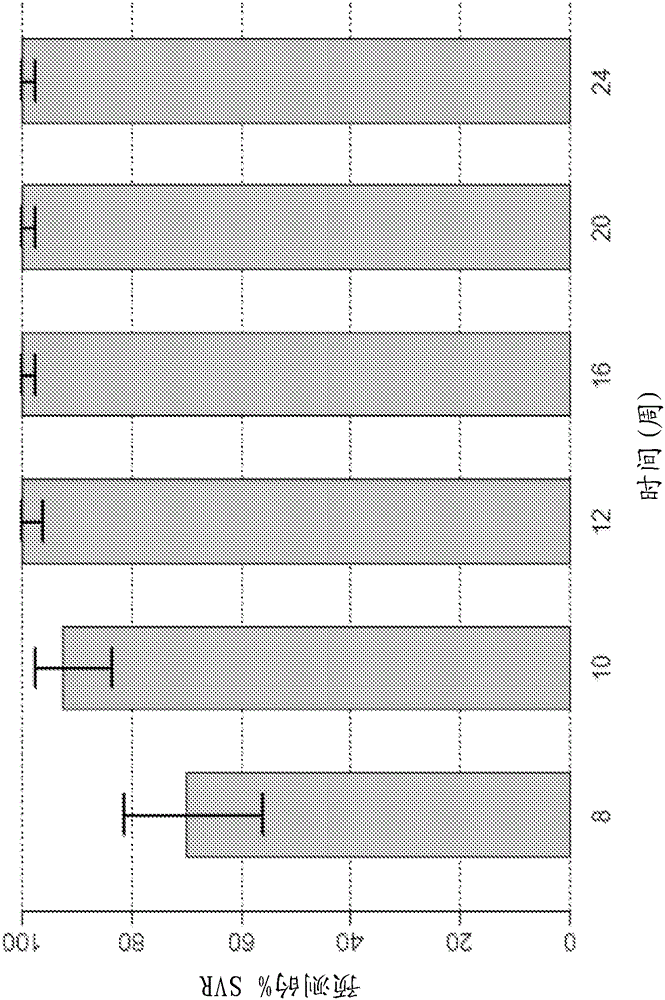
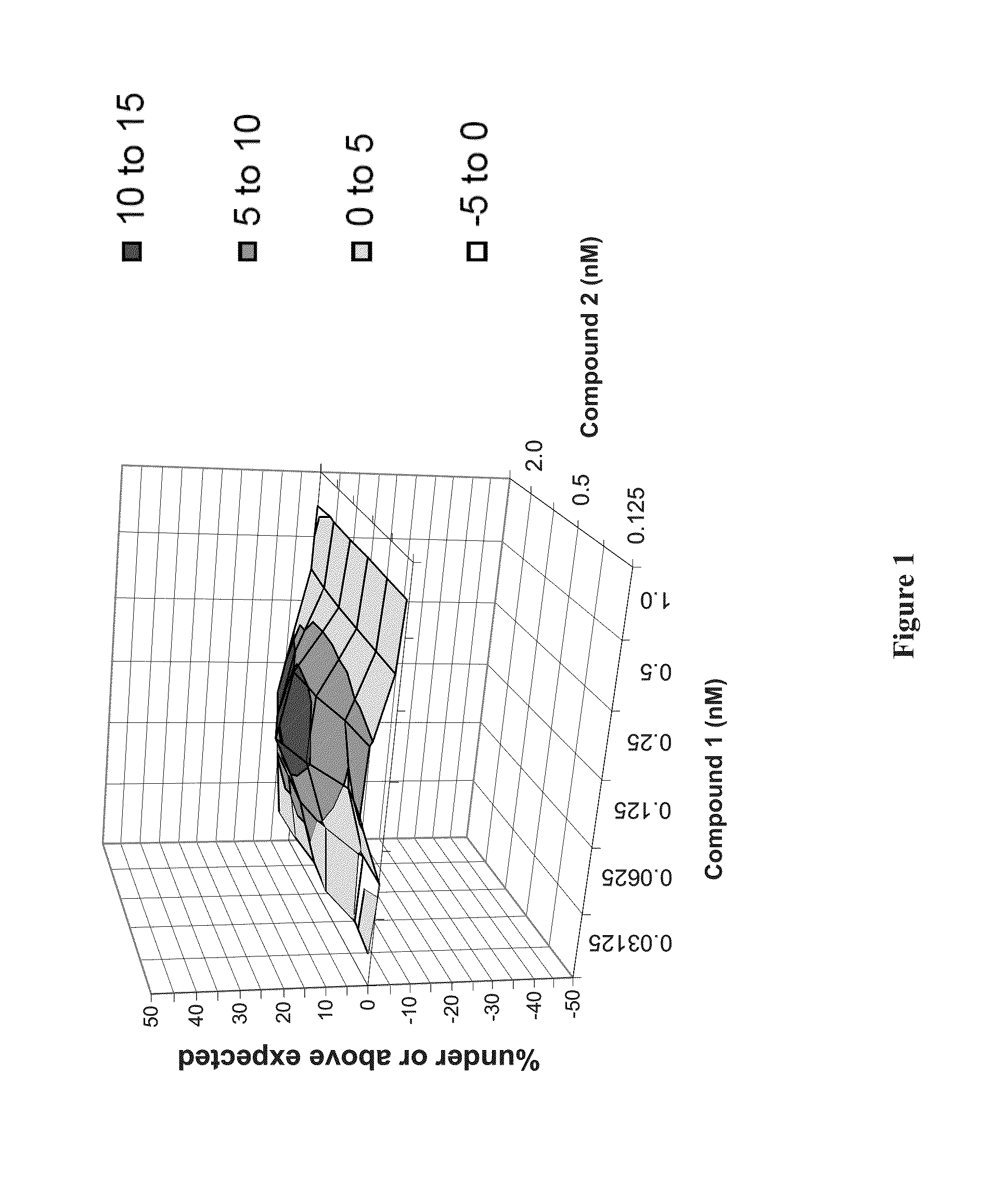
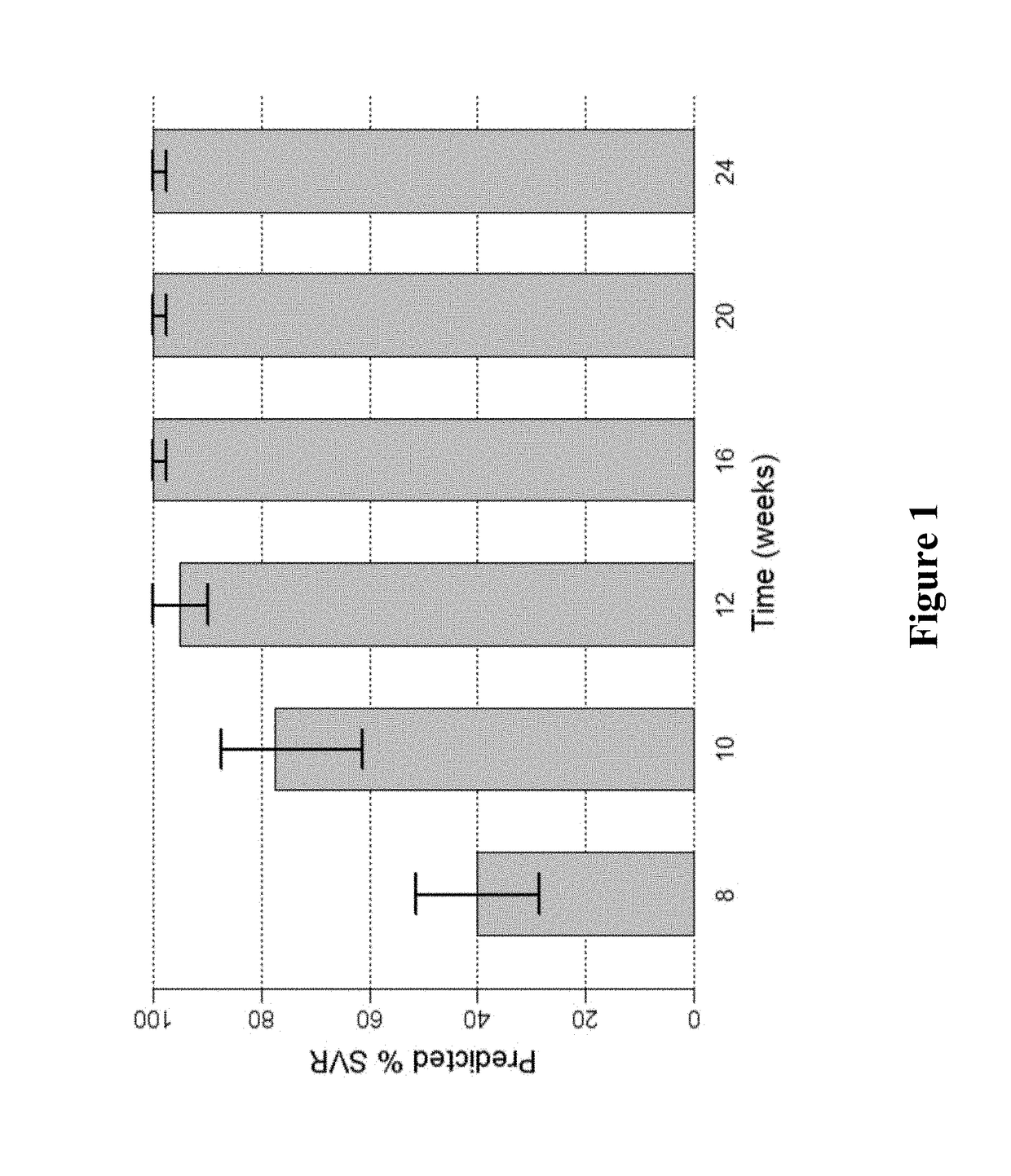
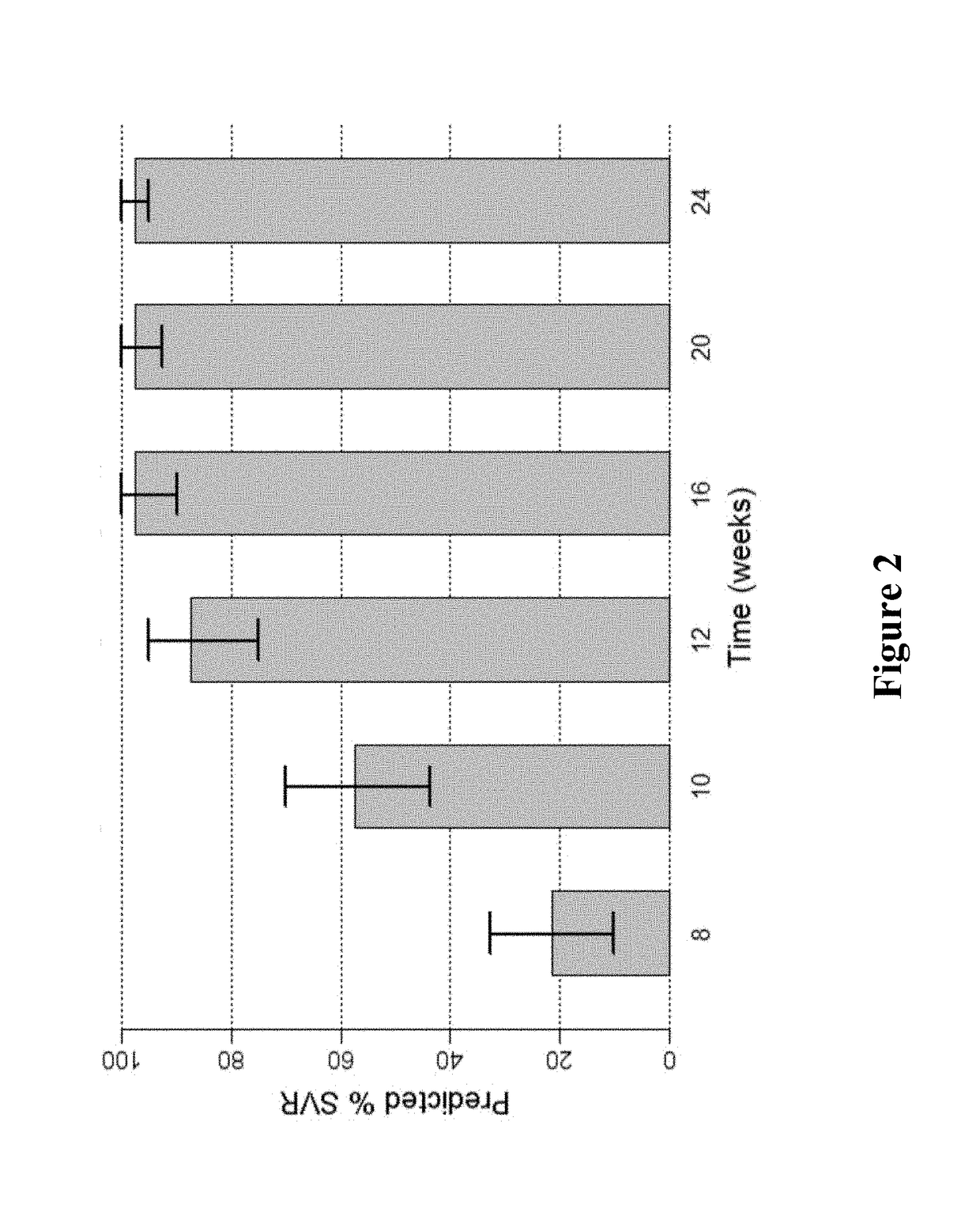
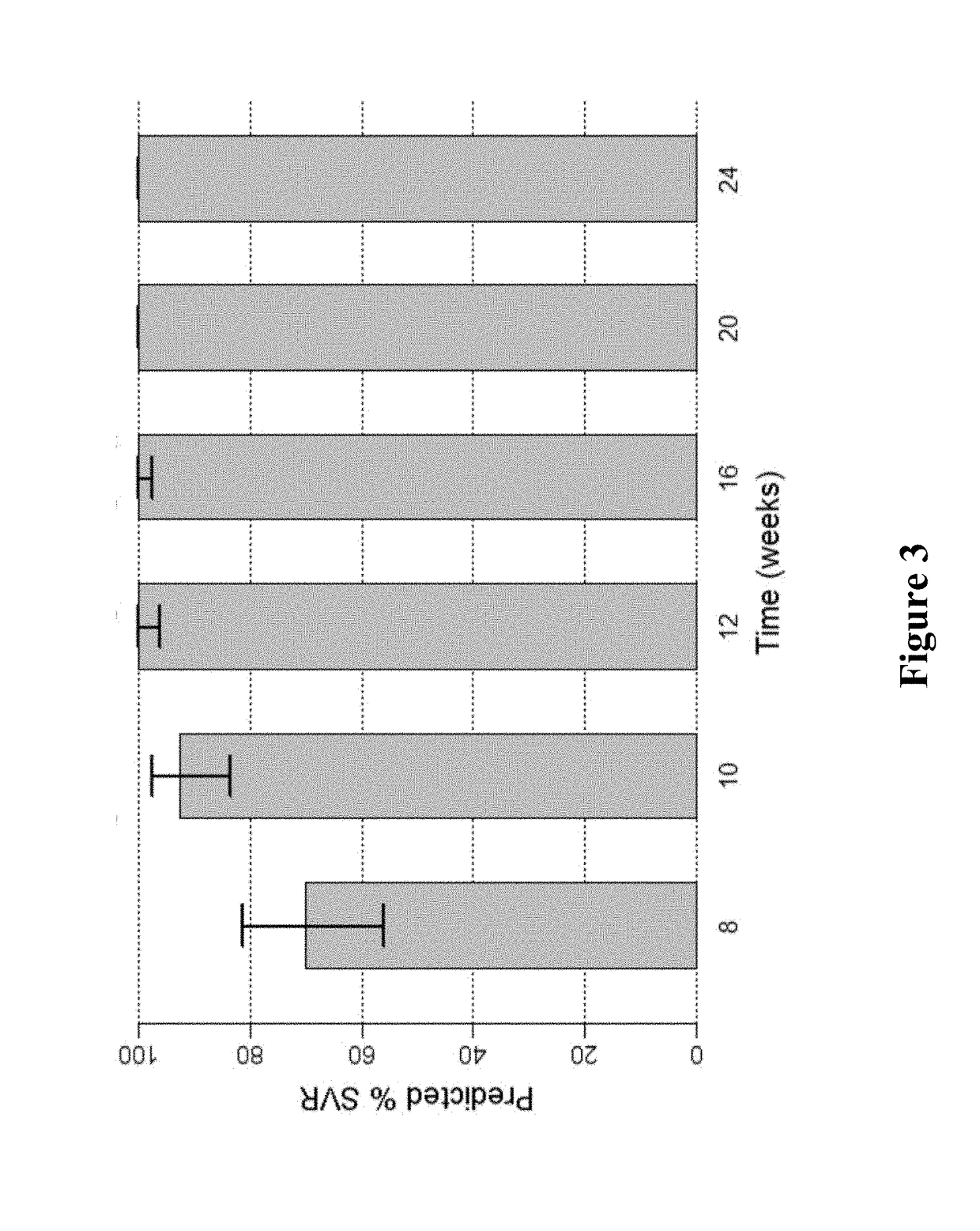
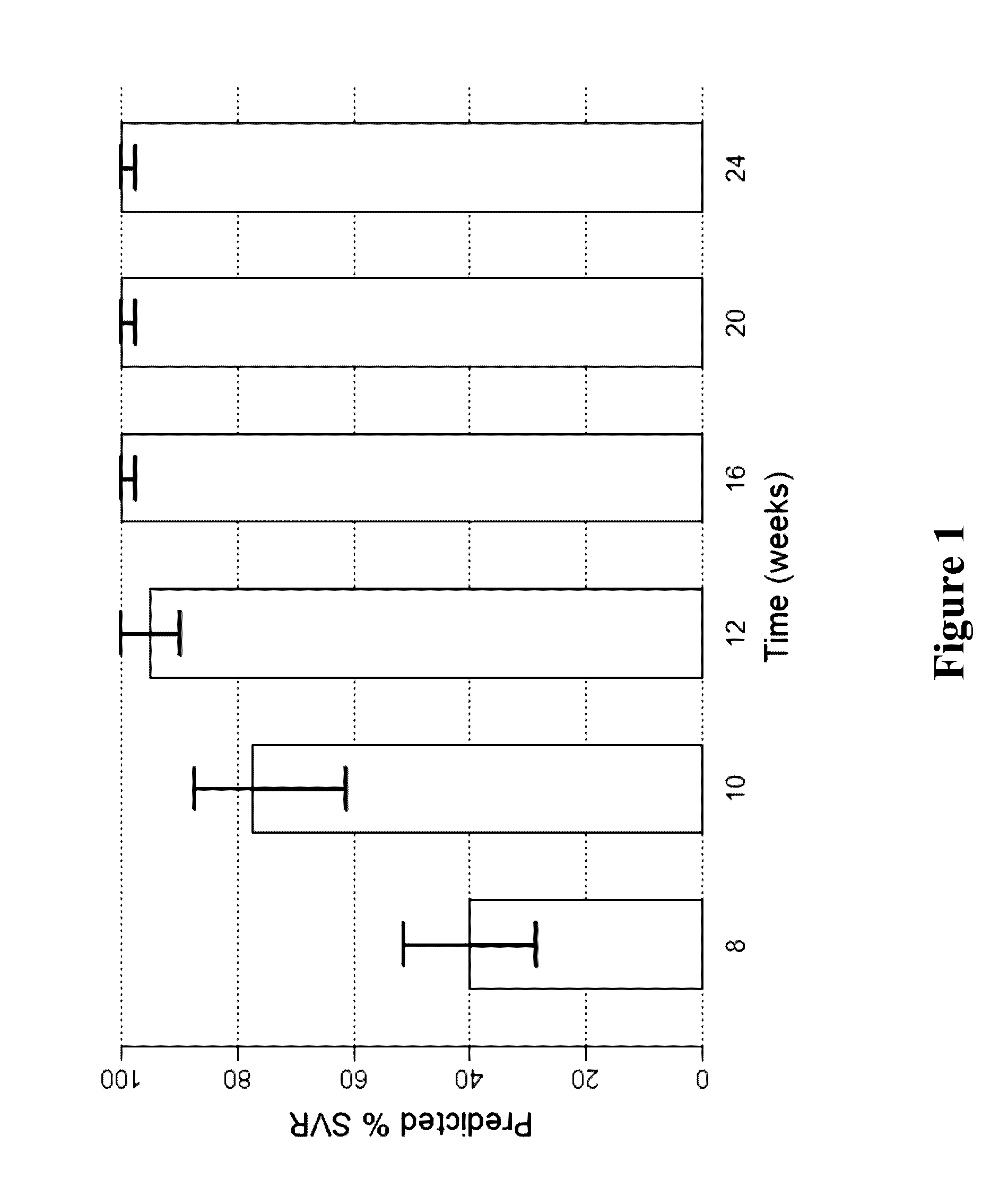
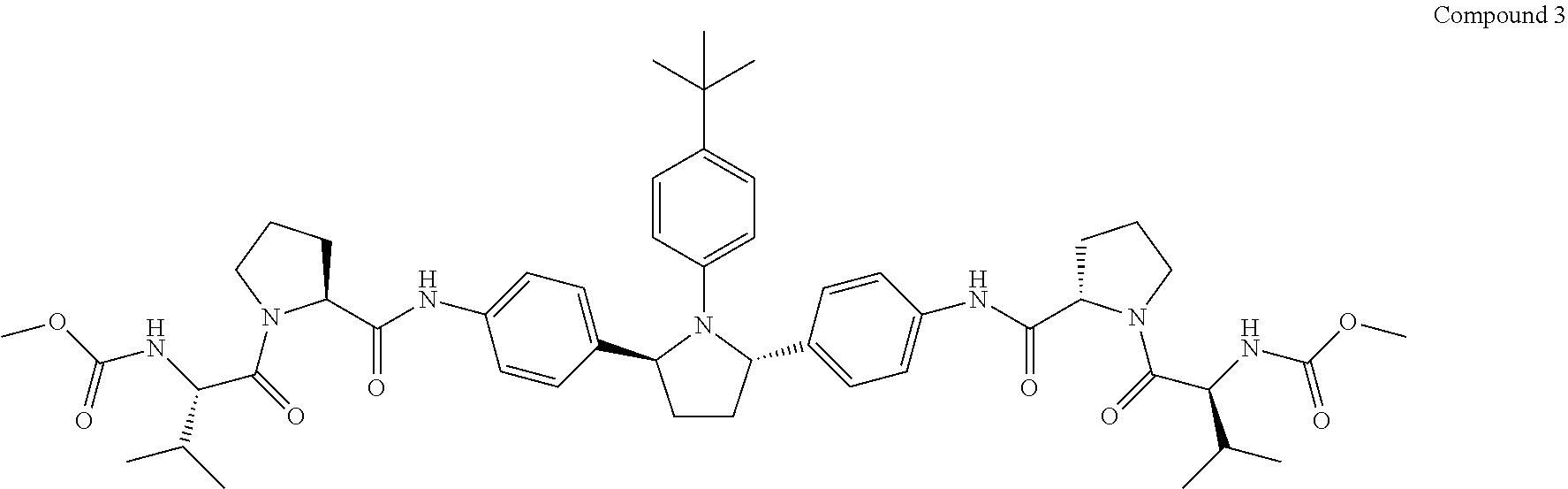
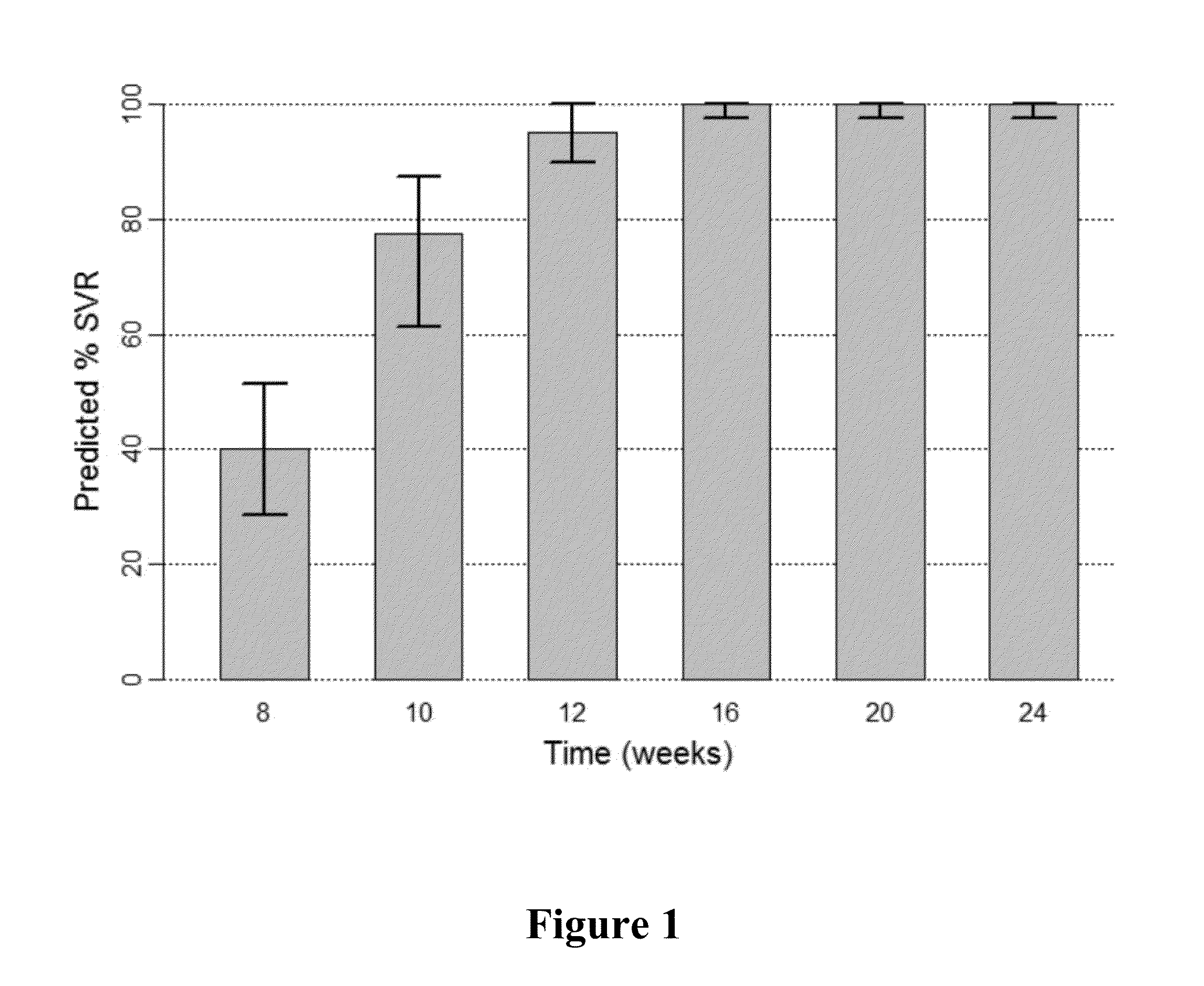
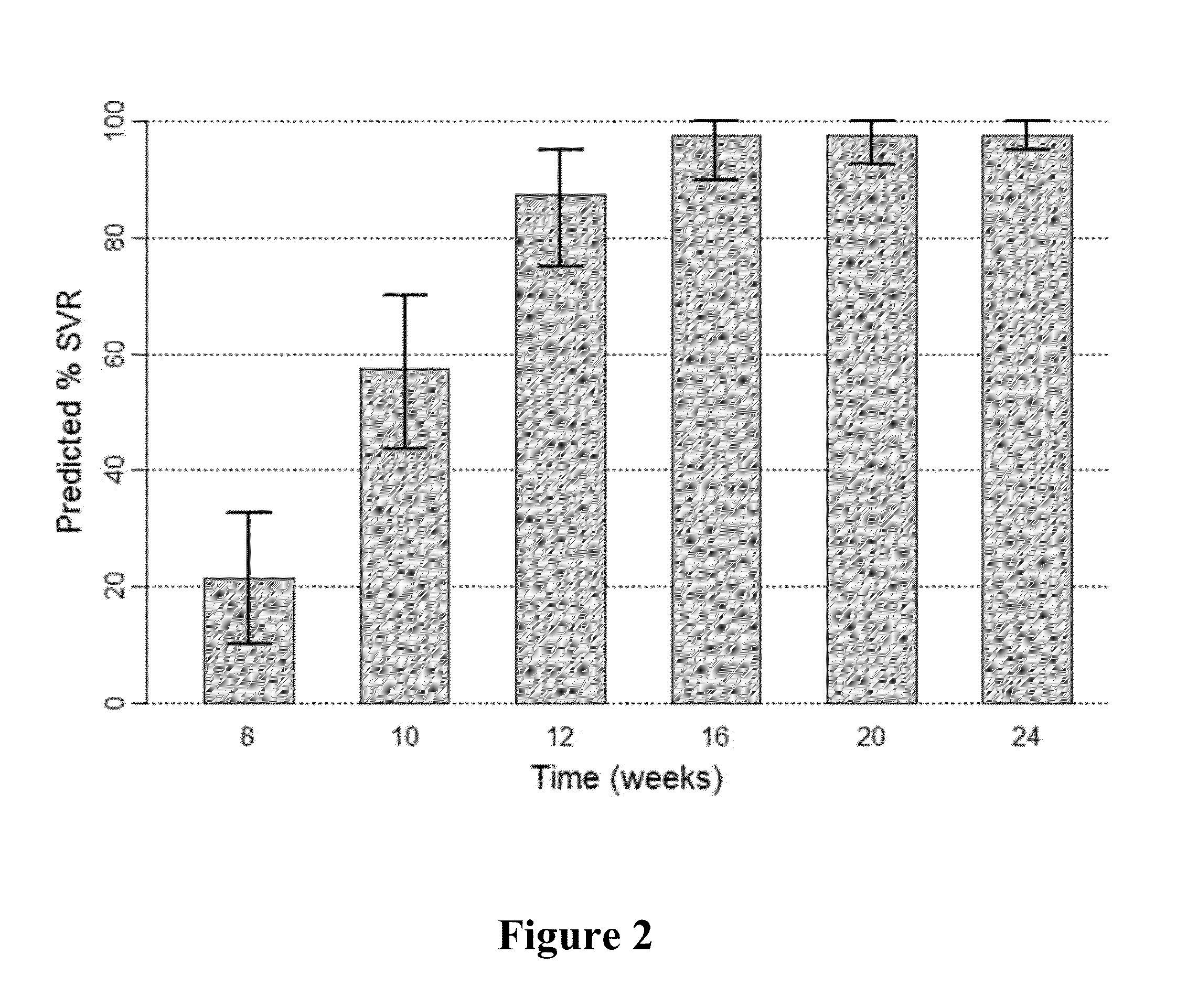
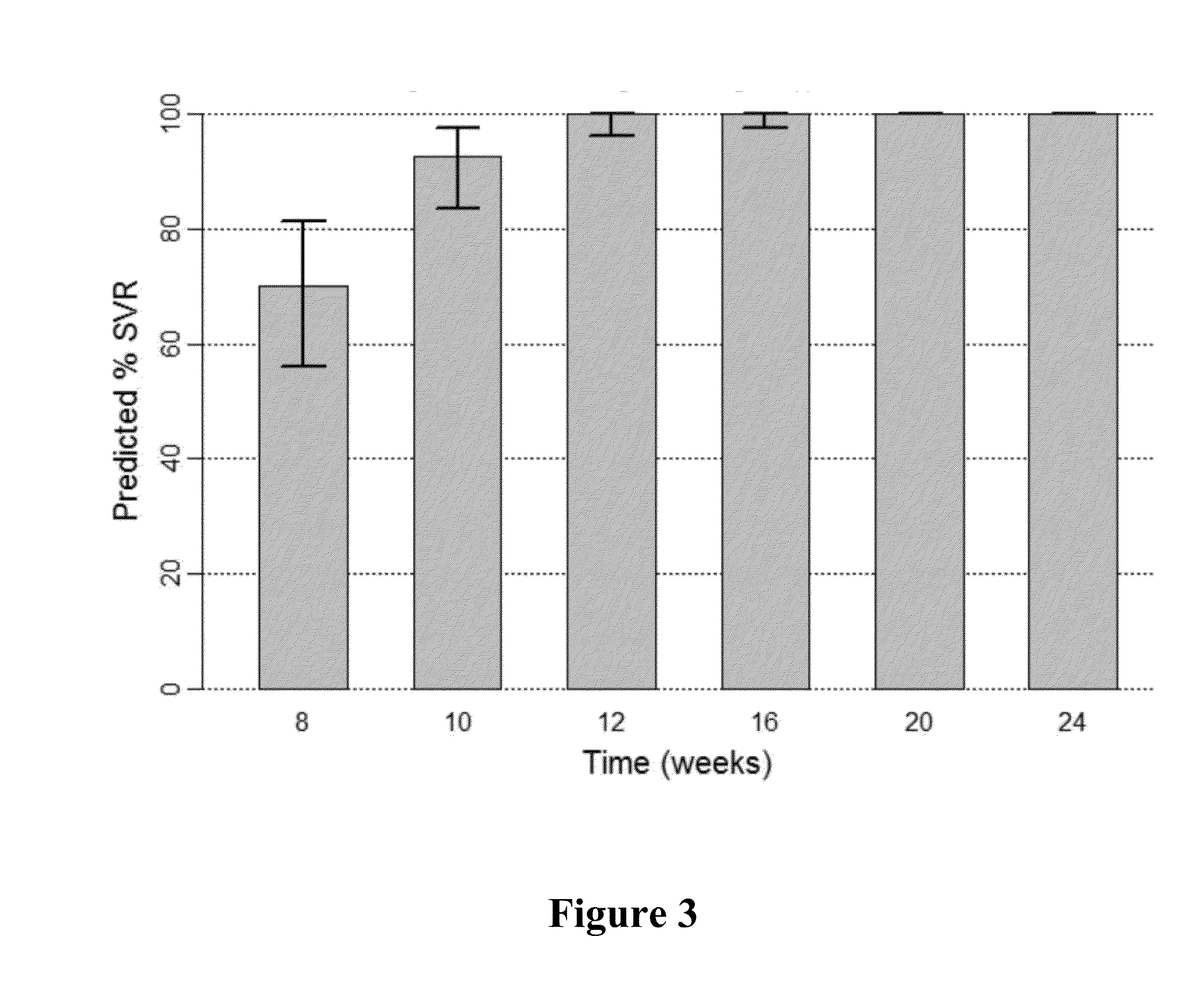
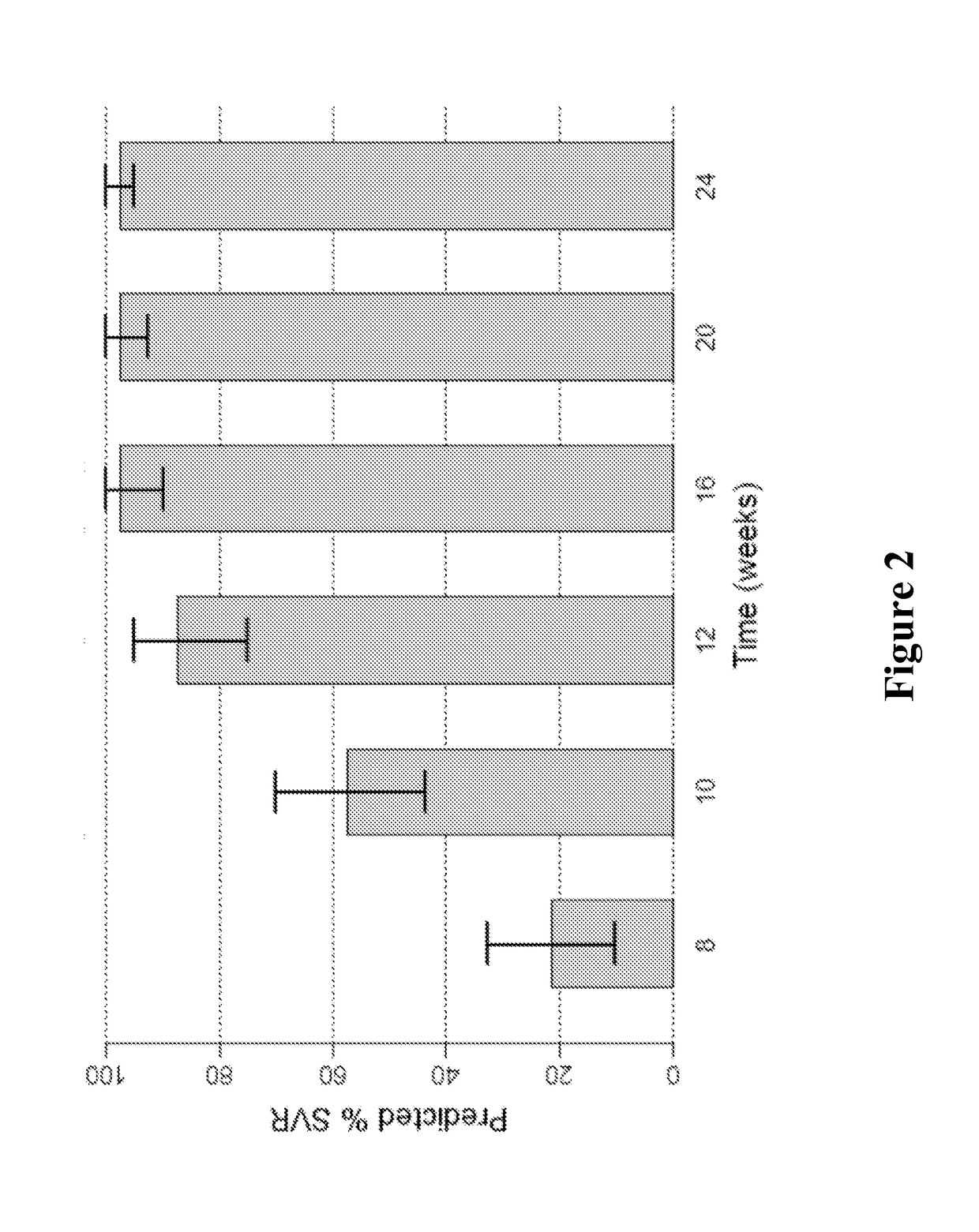
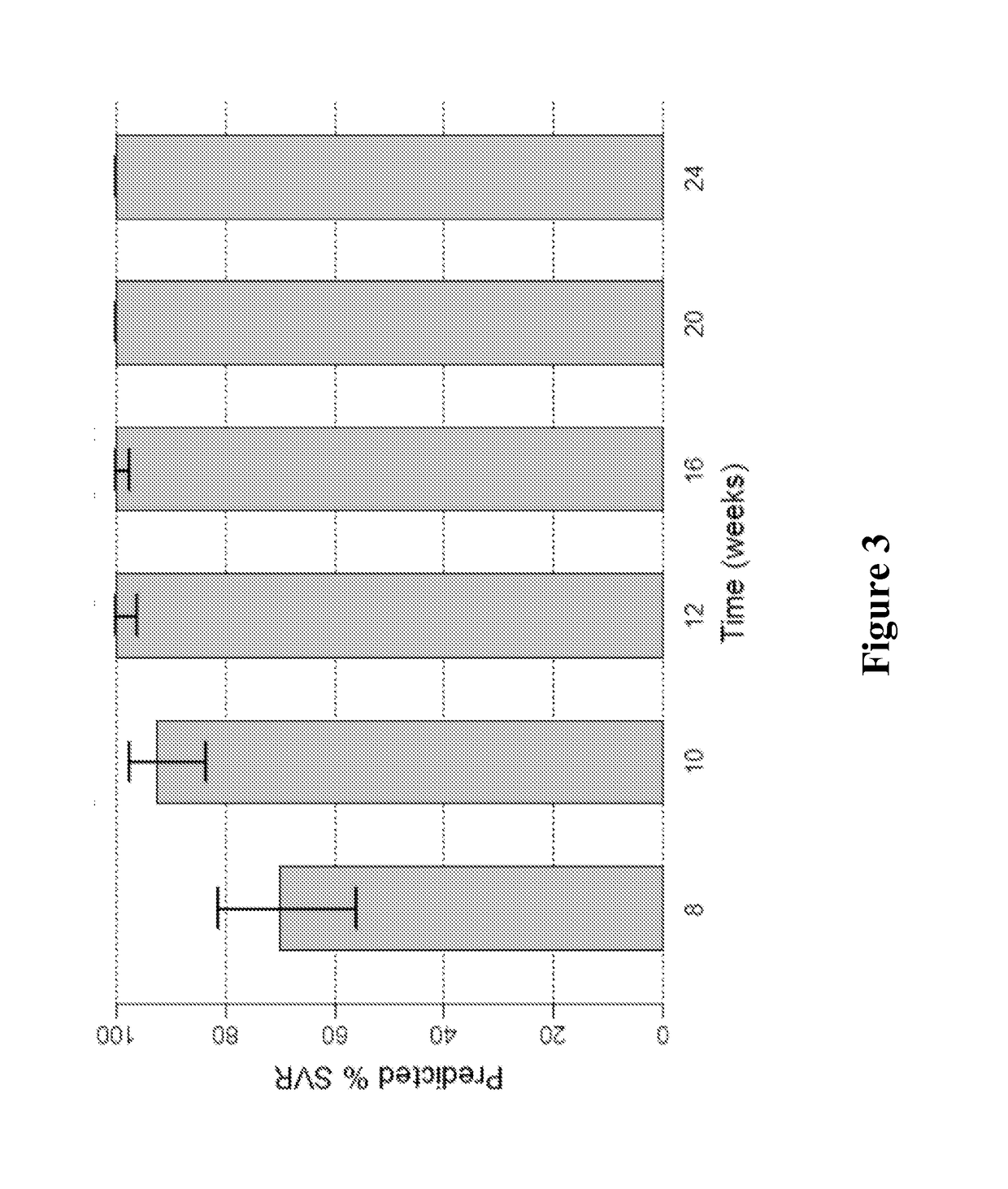
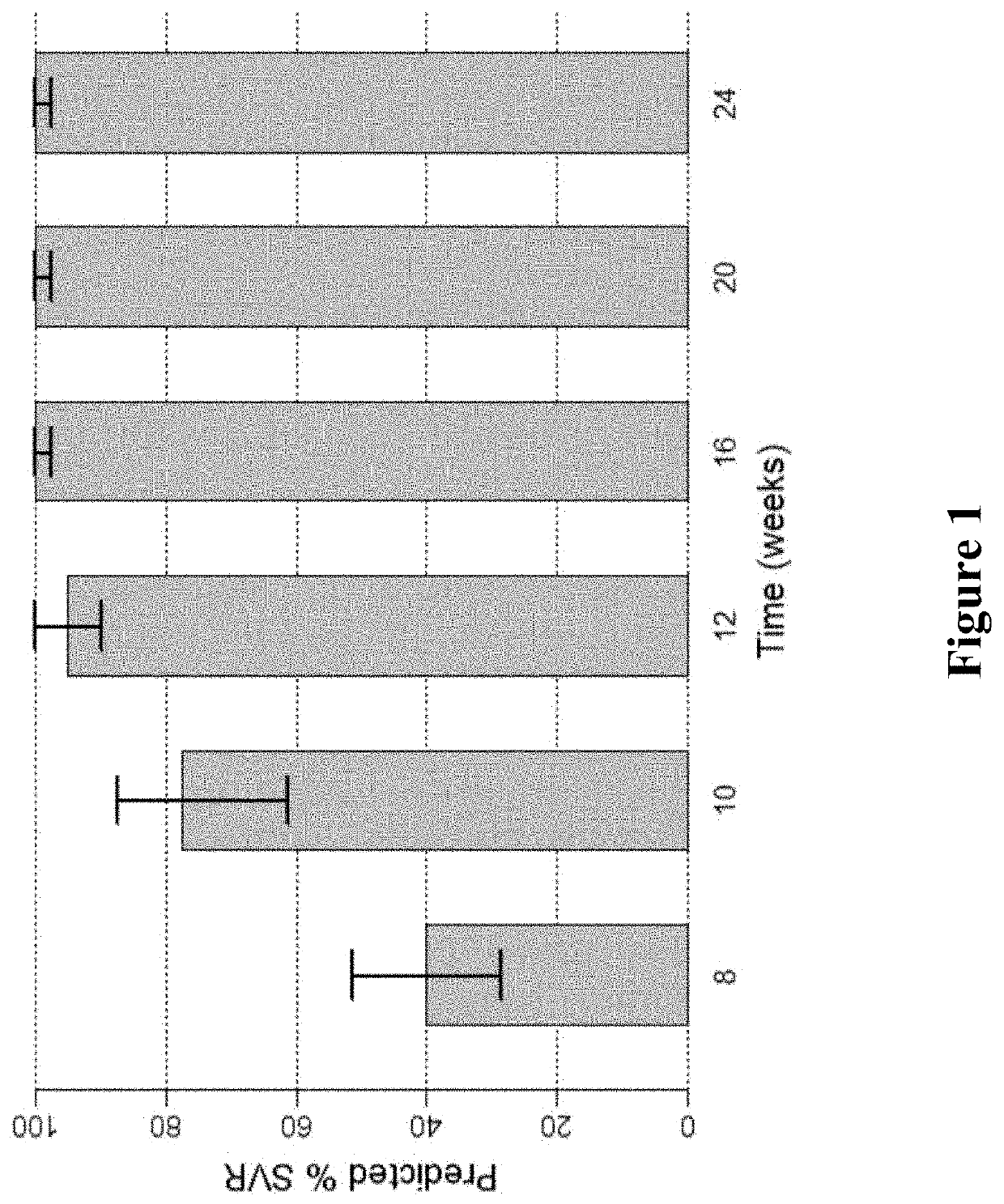
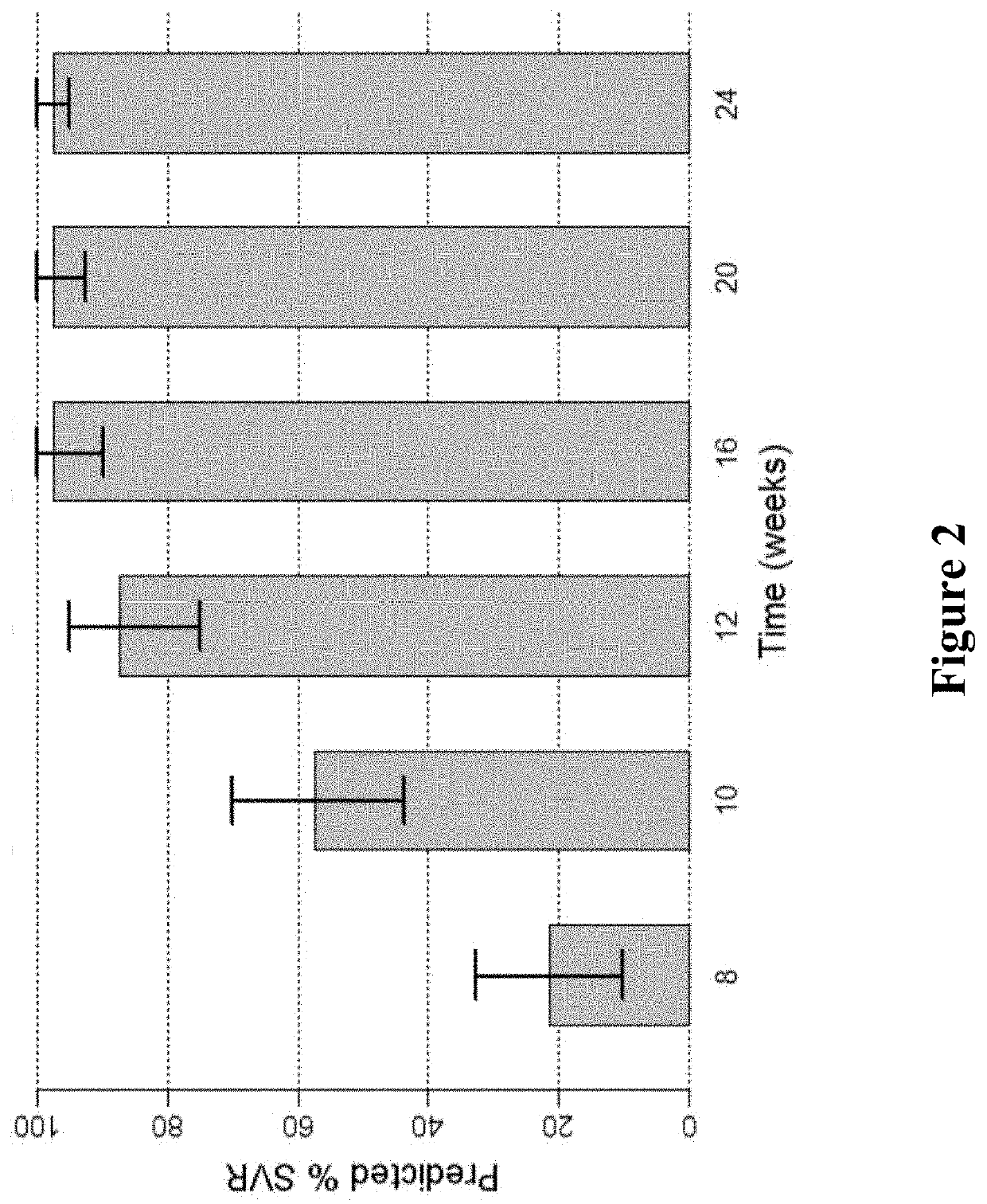
Patents
Literature
35 results about "DIRECT ACTING ANTIVIRALS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
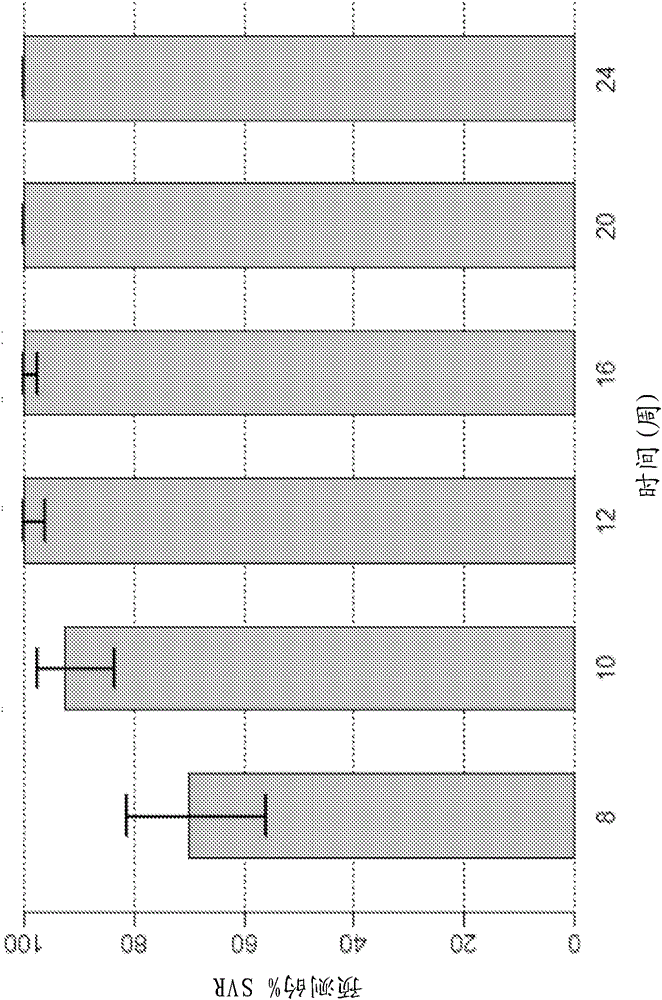
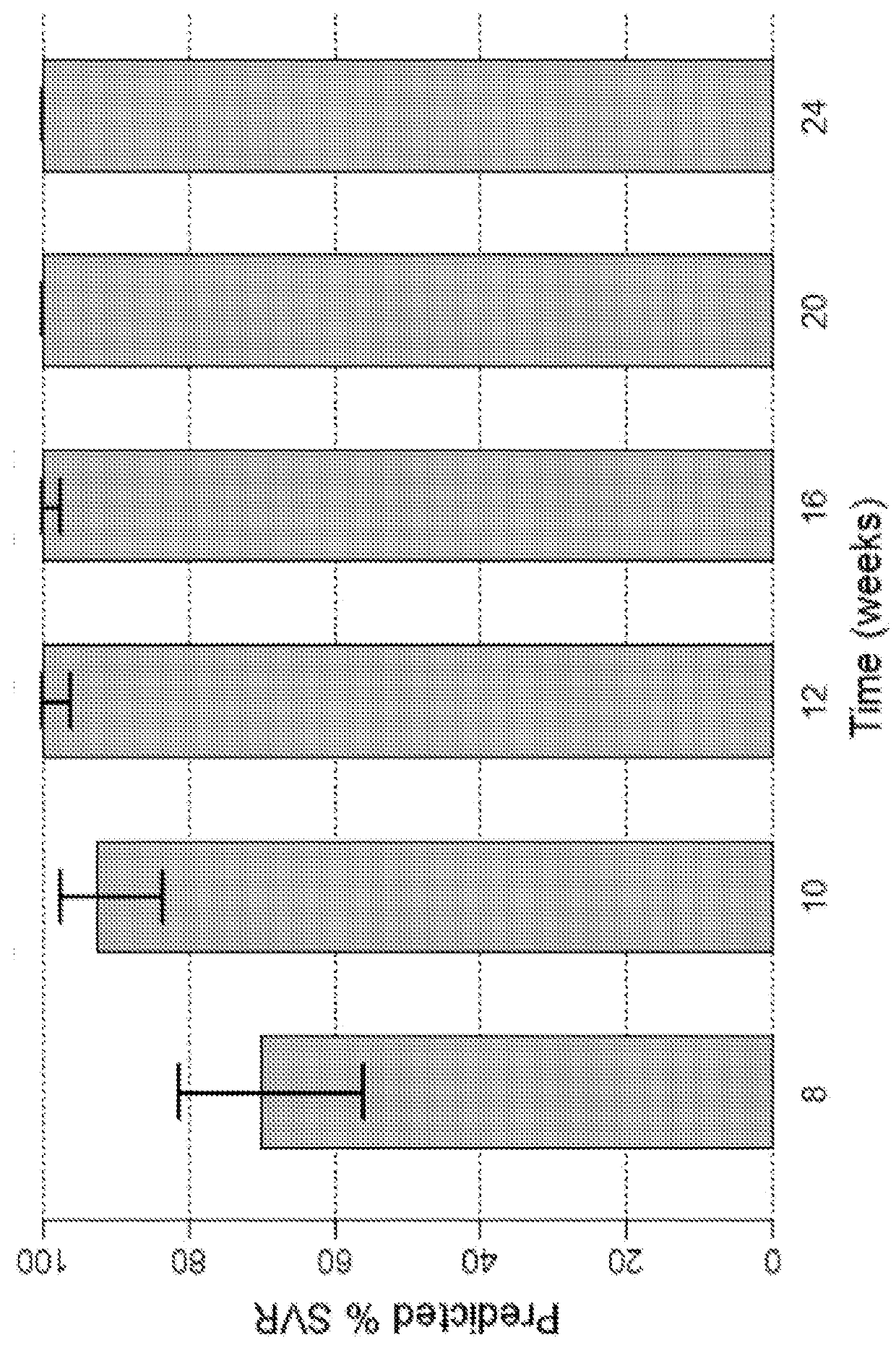
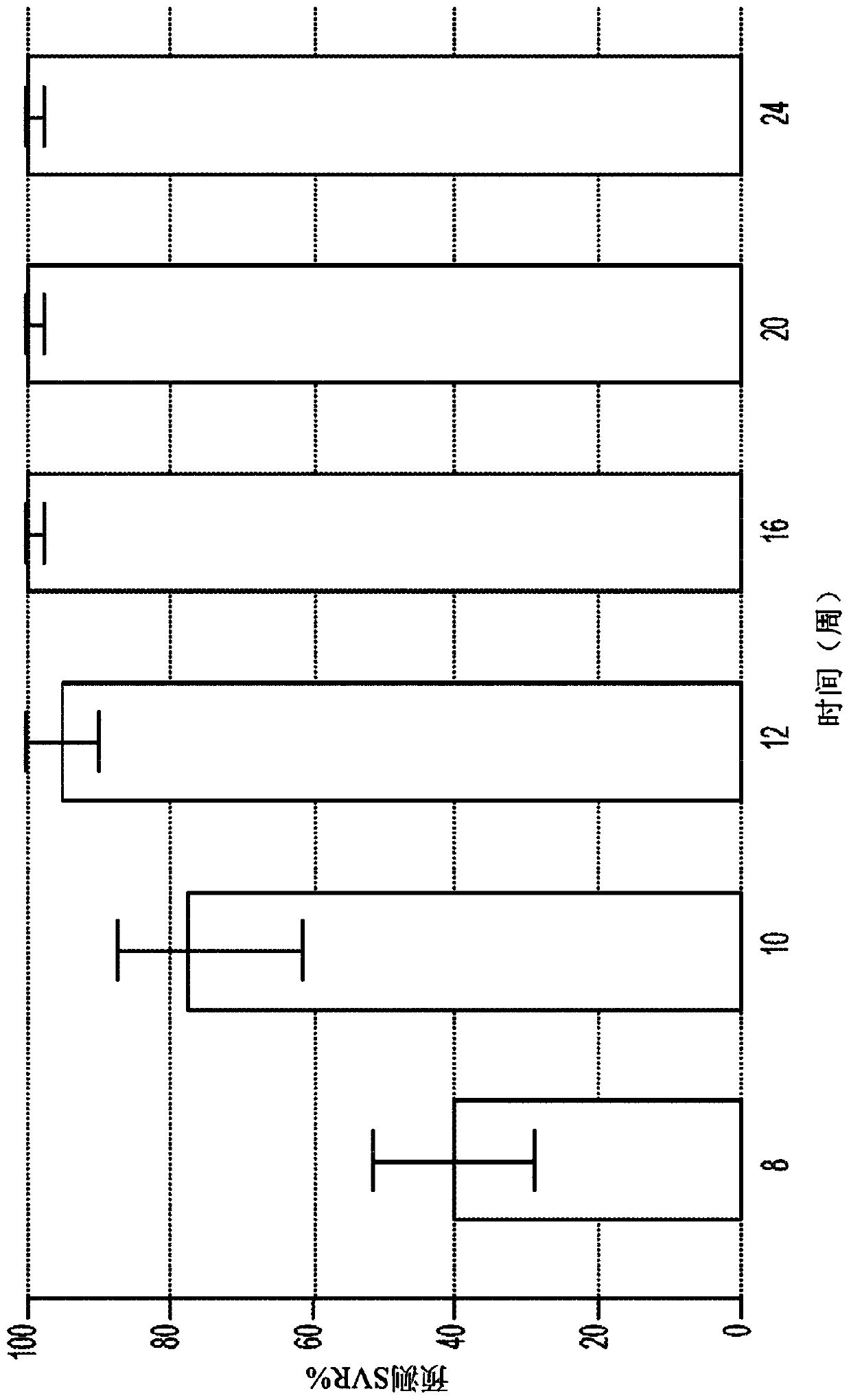
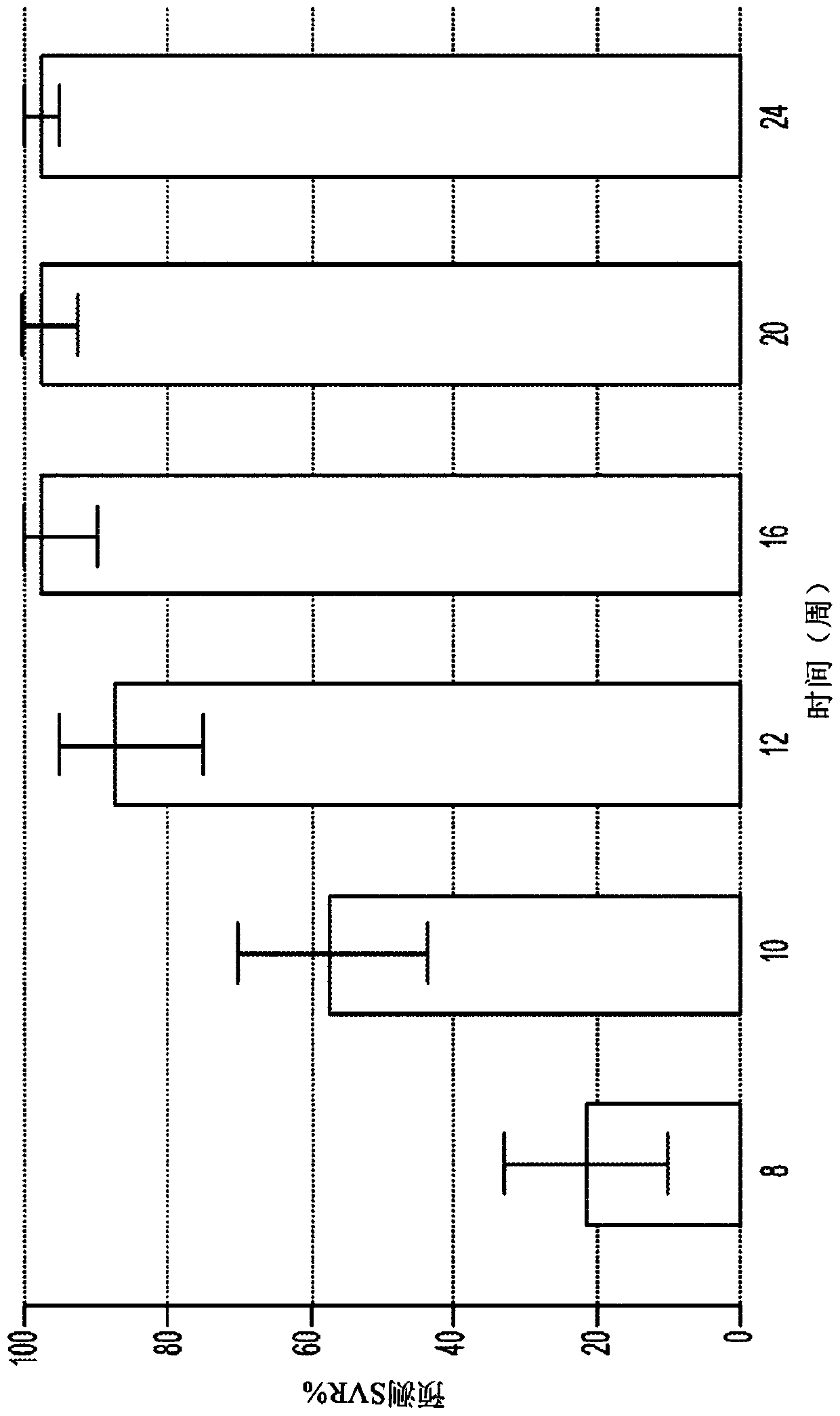
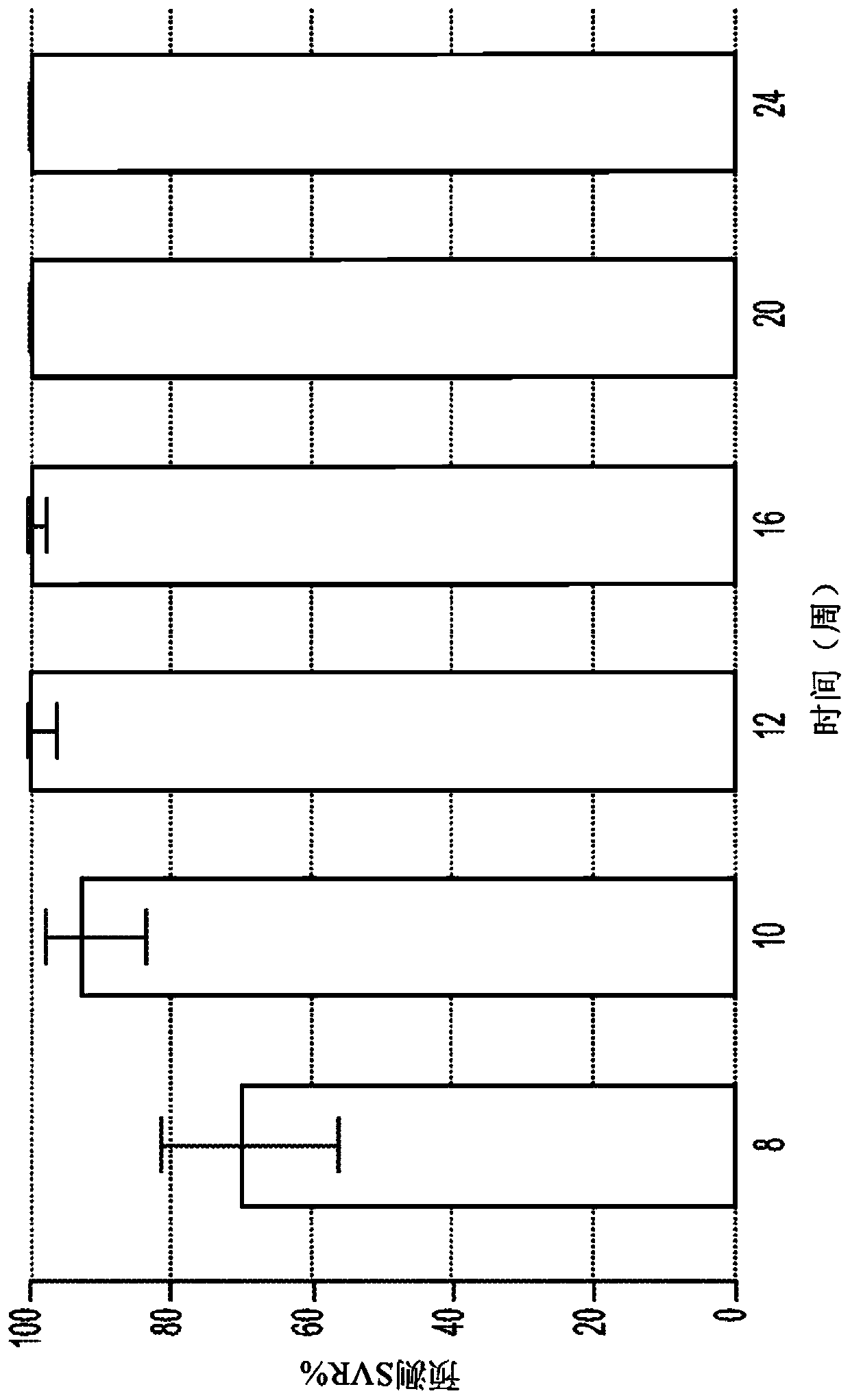
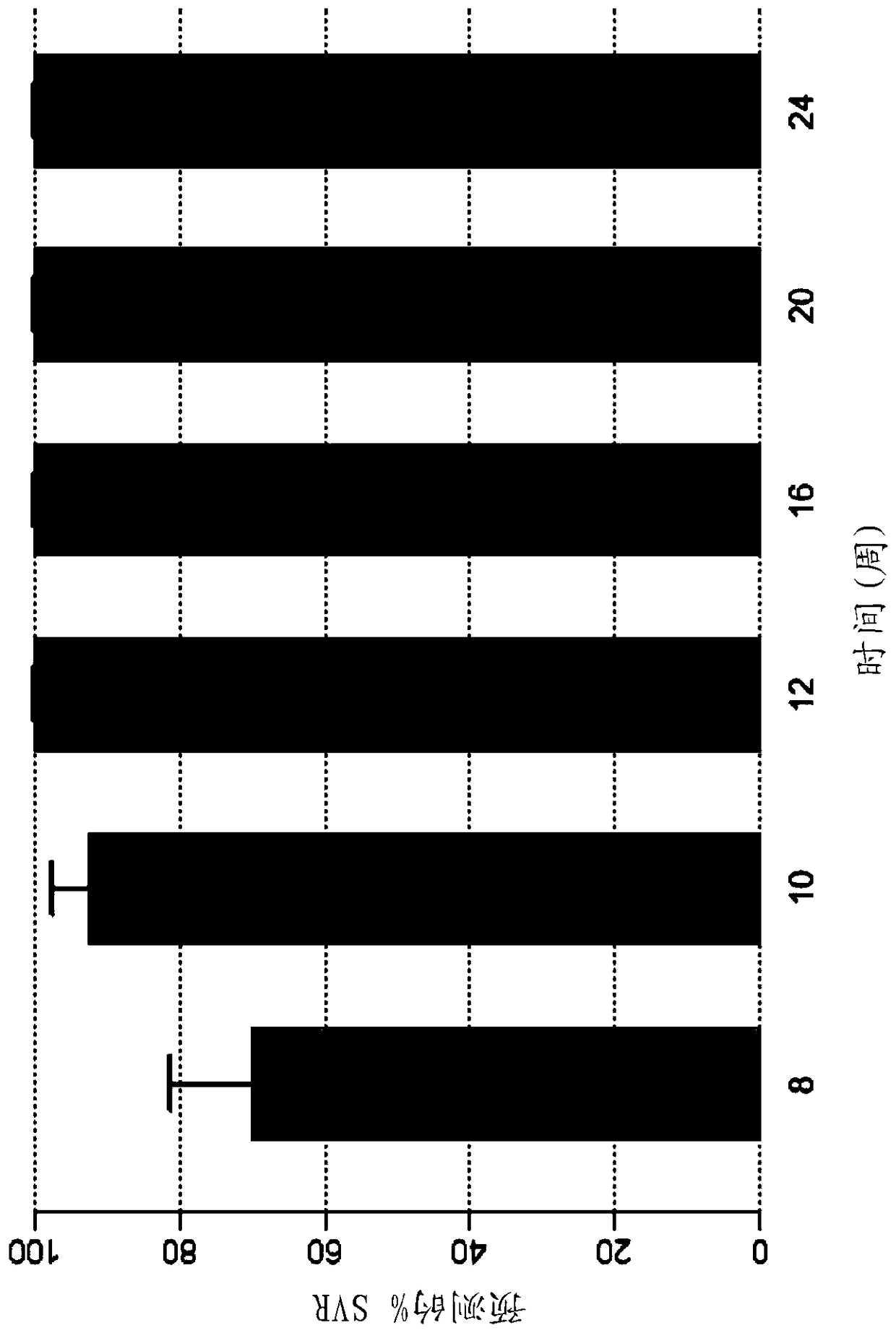
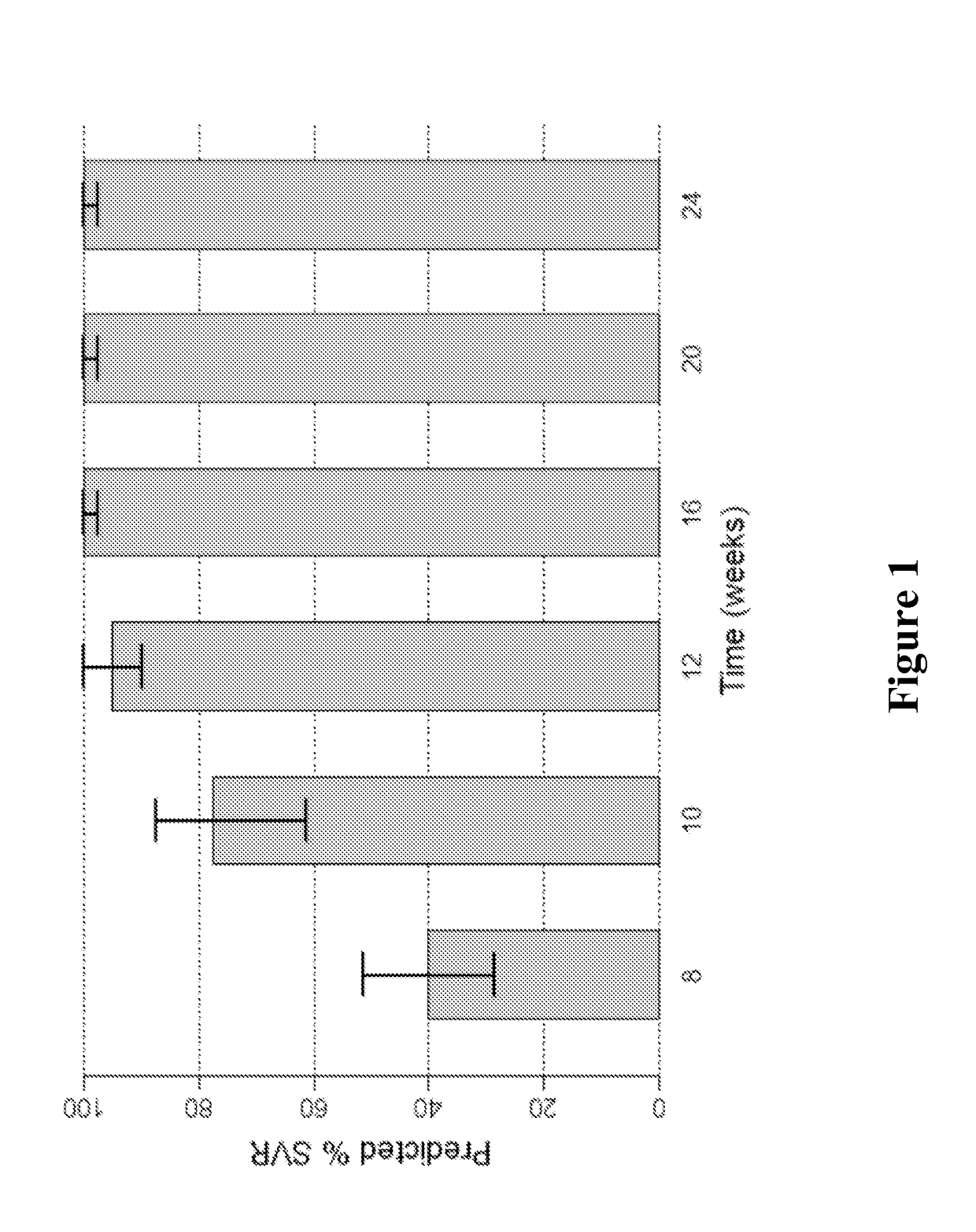
Direct-acting antivirals (DAAs) are a relatively new class of medication that acts to target specific steps in the HCV viral life cycle. The goals of DAAs are to shorten the length of therapy, minimize side effects, target the virus itself, and improve sustained virologic response (SVR) rate.
Methods for Treating HCV
ActiveUS20130102526A1Improve pharmacokineticsImprove bioavailabilityBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon- and ribavirin-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents without interferon and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to a subject an effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), and an inhibitor of cytochrome P450 (e.g., ritonavir).
Owner:ABBVIE INC
Methods for Treating HCV
ActiveUS20130102525A1Avoid side effectsImprove pharmacokineticsBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to the subject effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), an inhibitor of cytochrome P450 (e.g., ritonavir), and ribavirin.
Owner:ABBVIE INC
Methods for treating HCV
ActiveUS8466159B2Improve pharmacokineticsImprove bioavailabilityBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to the subject effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), an inhibitor of cytochrome P450 (e.g., ritonavir), and ribavirin.
Owner:ABBVIE INC
Methods for treating HCV
ActiveUS8492386B2Improve pharmacokineticsImprove bioavailabilityBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon- and ribavirin-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents without interferon and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to a subject an effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), and an inhibitor of cytochrome P450 (e.g., ritonavir).
Owner:ABBVIE INC
Methods for treating HCV
ActiveUS8853176B2Improve pharmacokineticsImprove bioavailabilityBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to the subject effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), an inhibitor of cytochrome P450 (e.g., ritonavir), and ribavirin.
Owner:ABBVIE INC
Methods for treating hcv
InactiveUS20140275099A1Avoid side effectsEfficient managementBiocideDigestive systemShort durationInterferon alpha
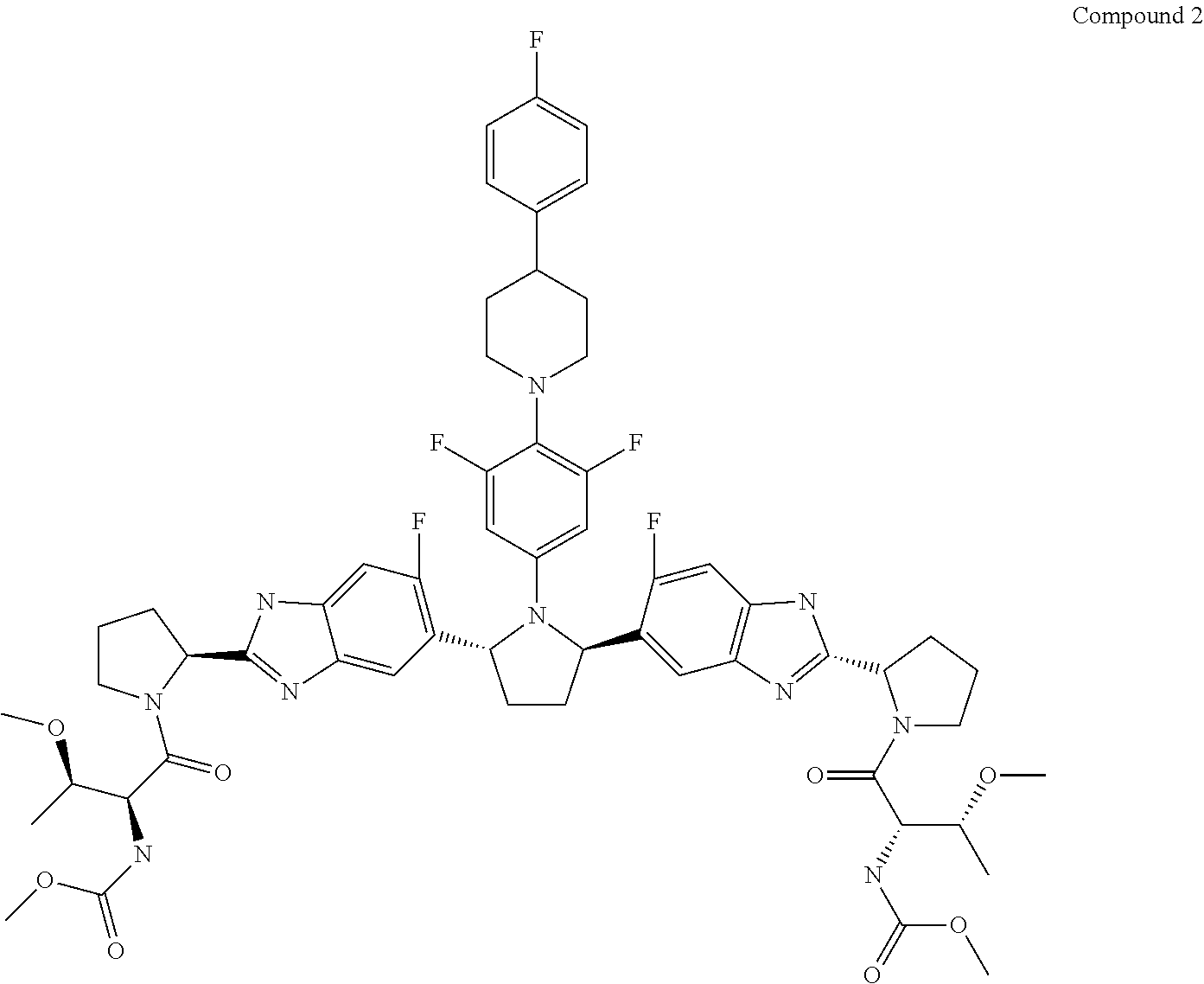
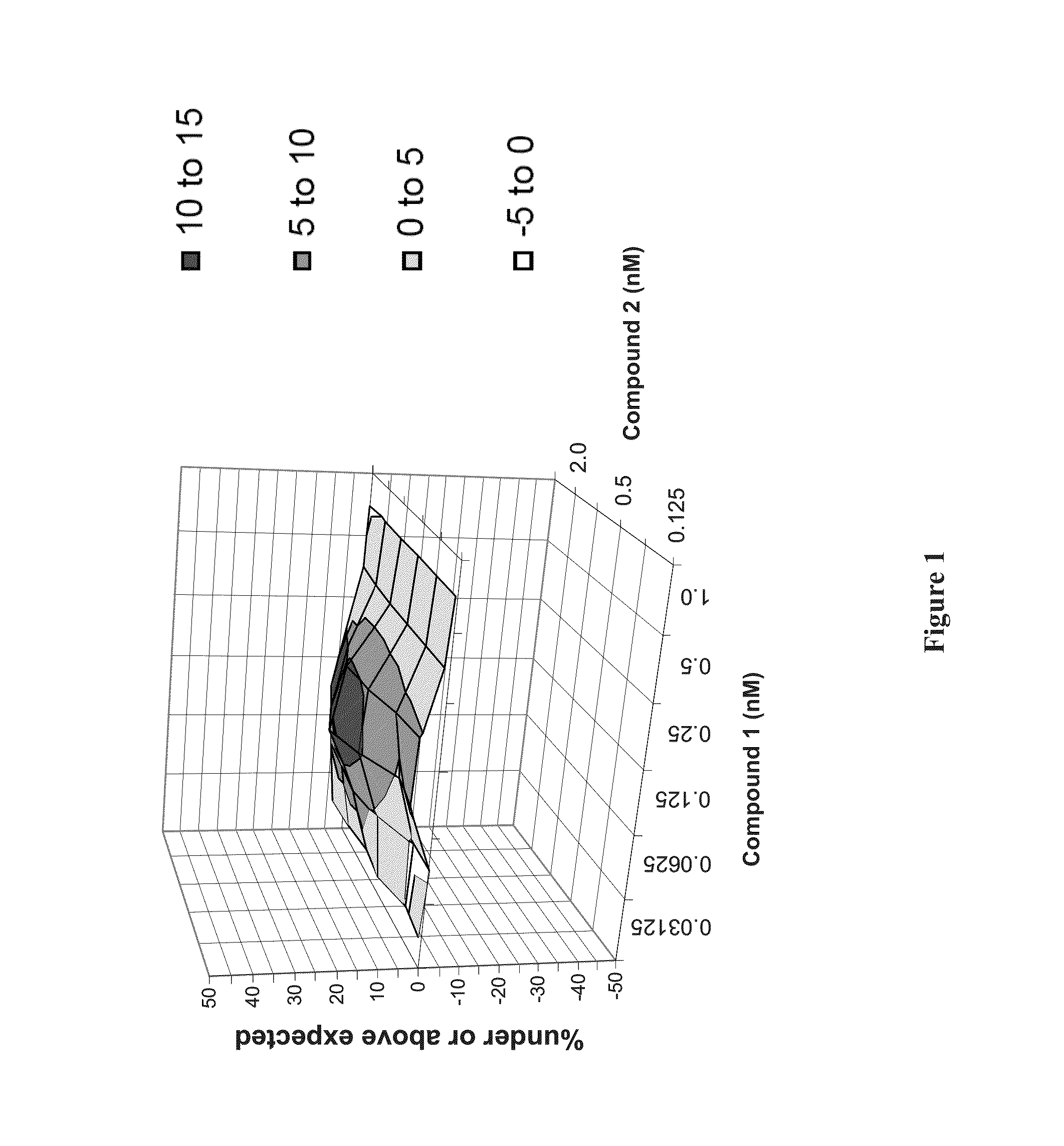
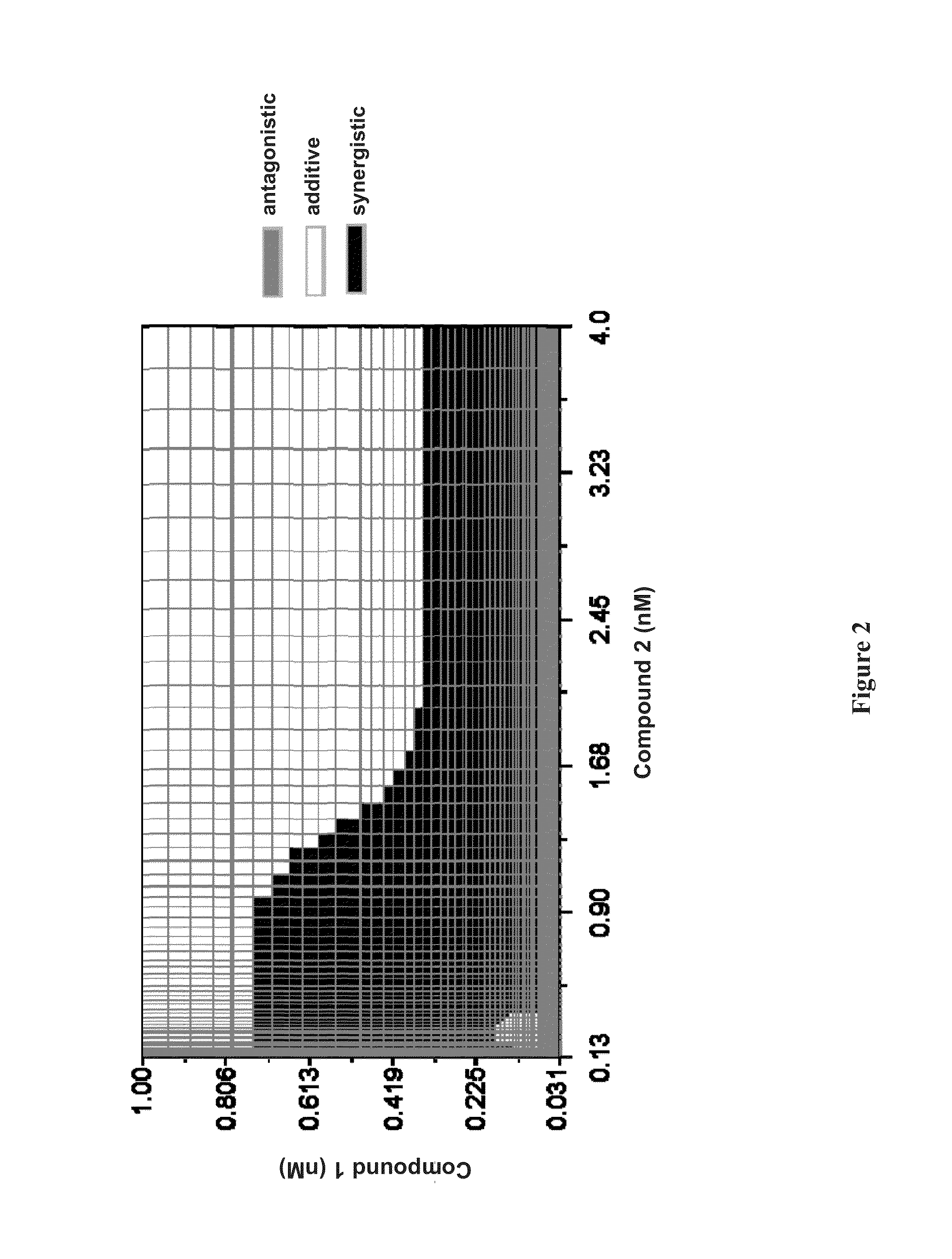
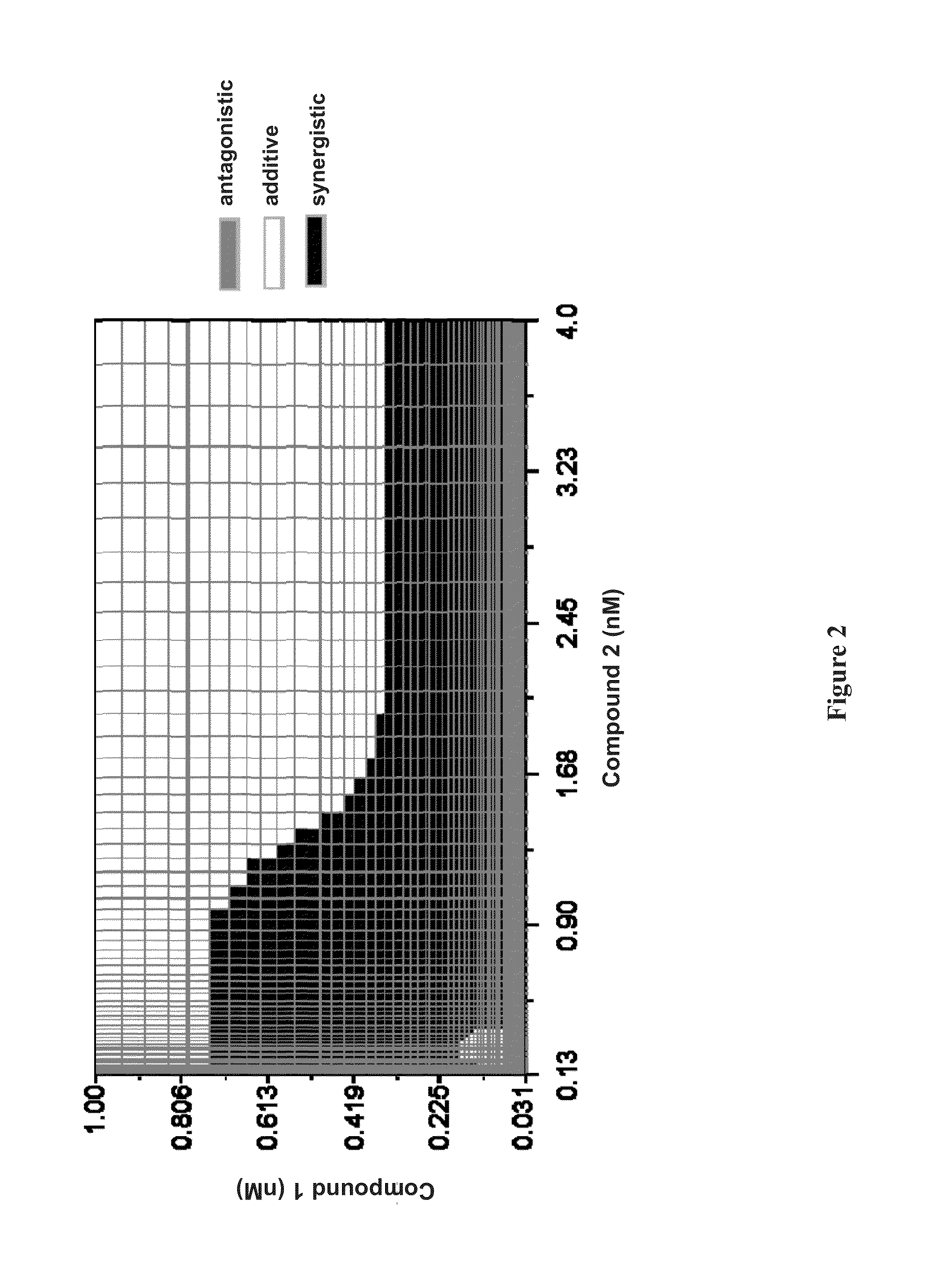
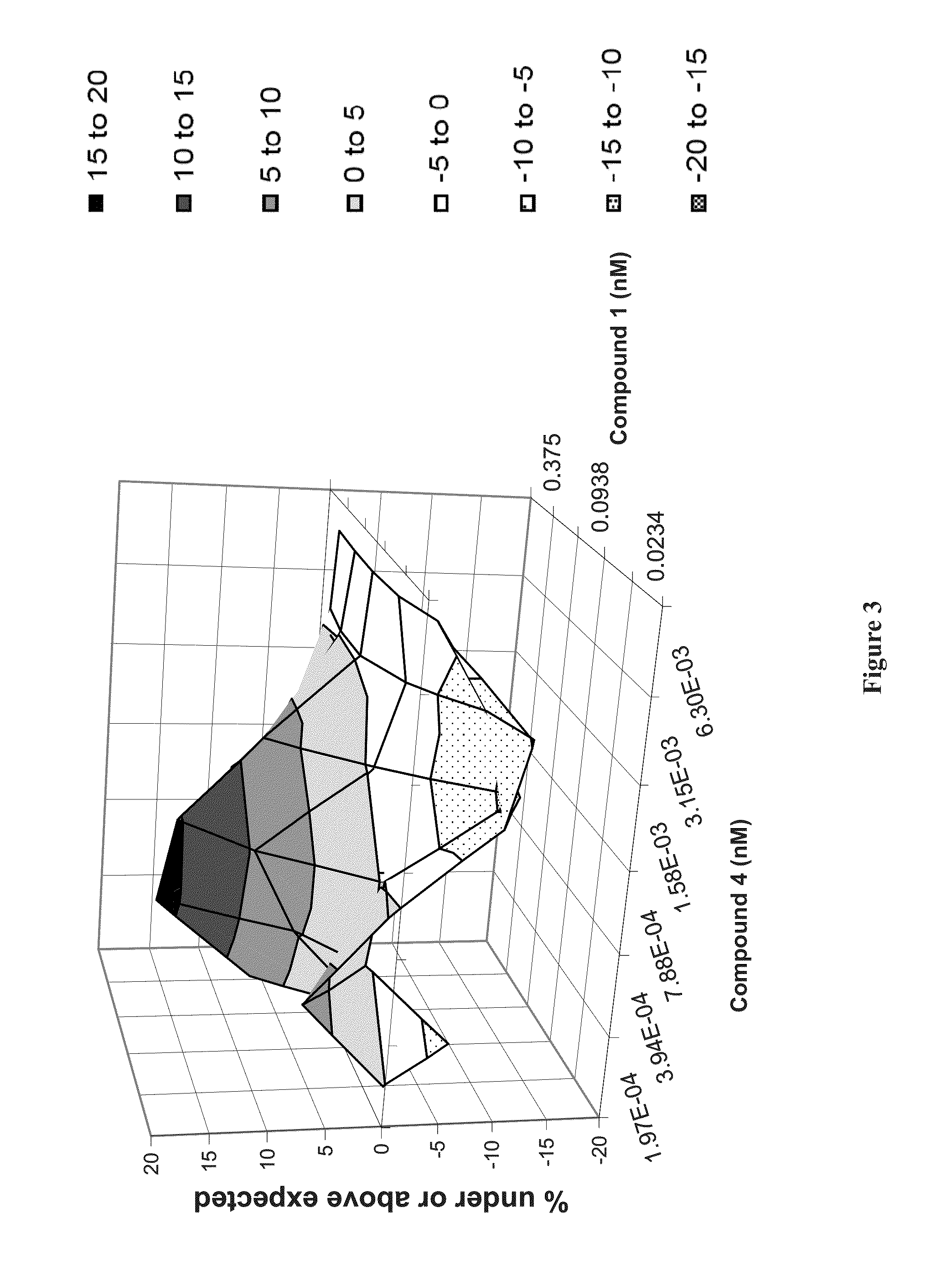
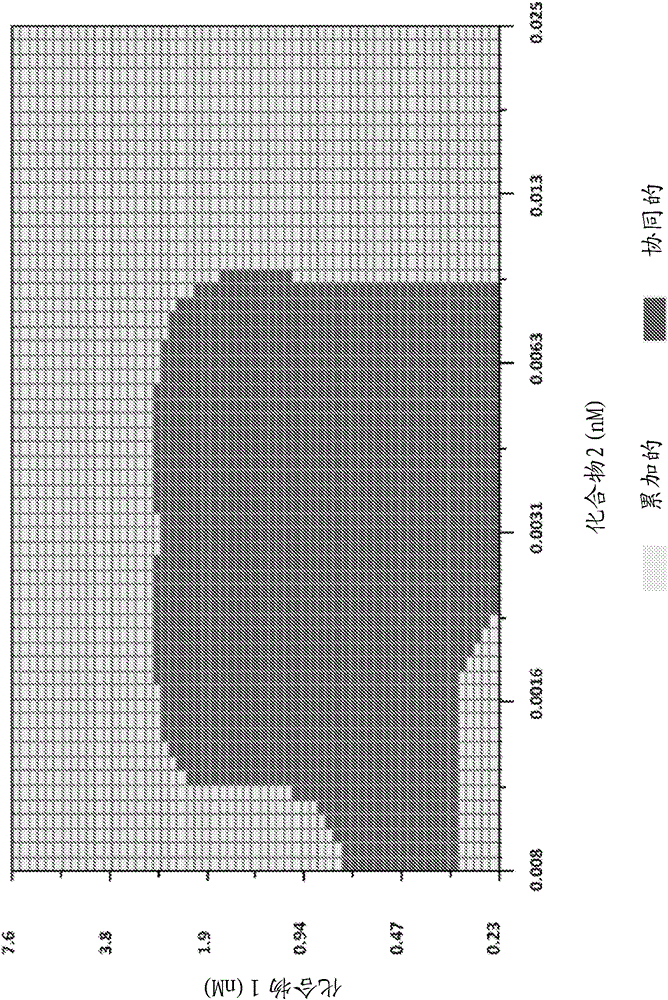
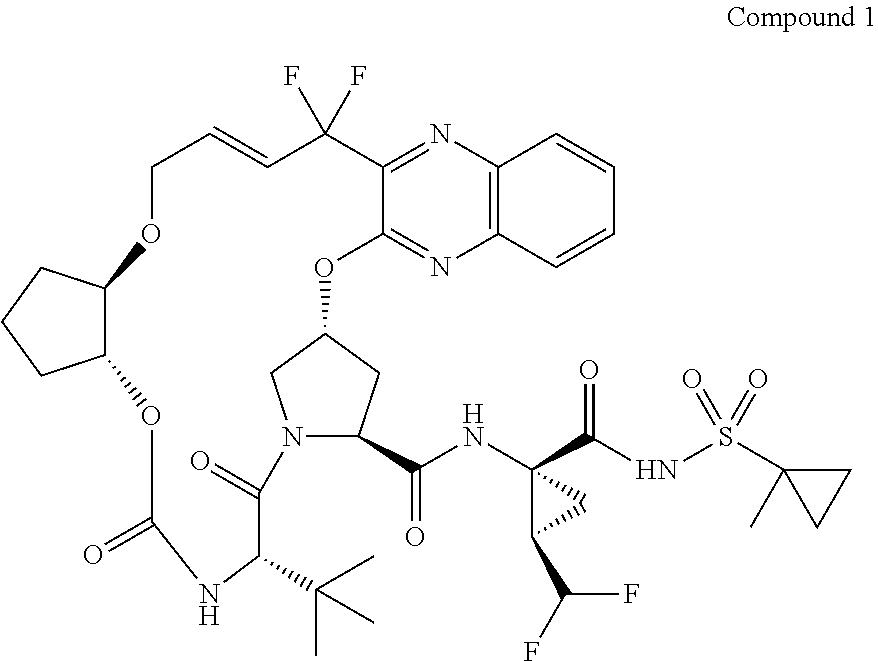
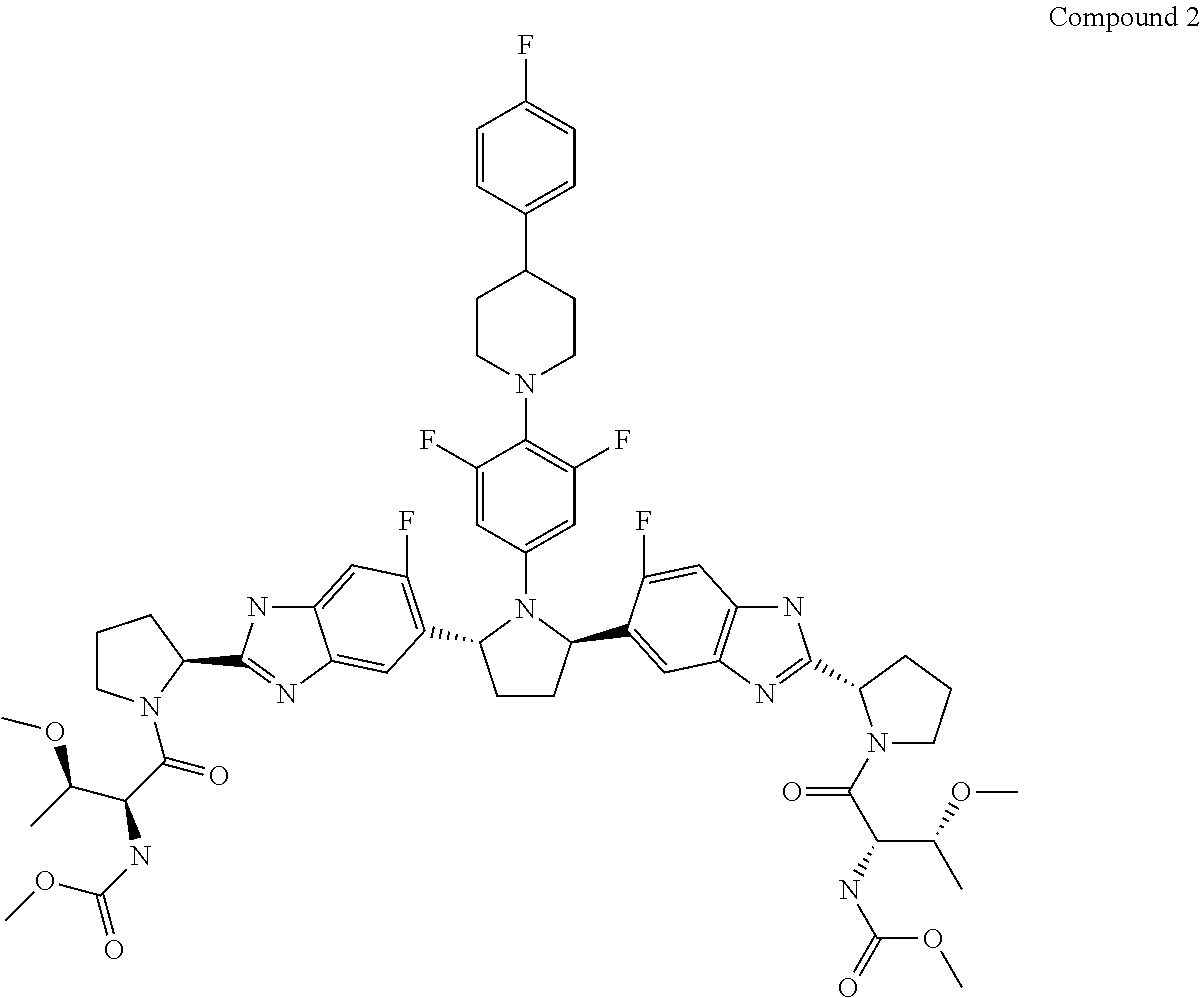
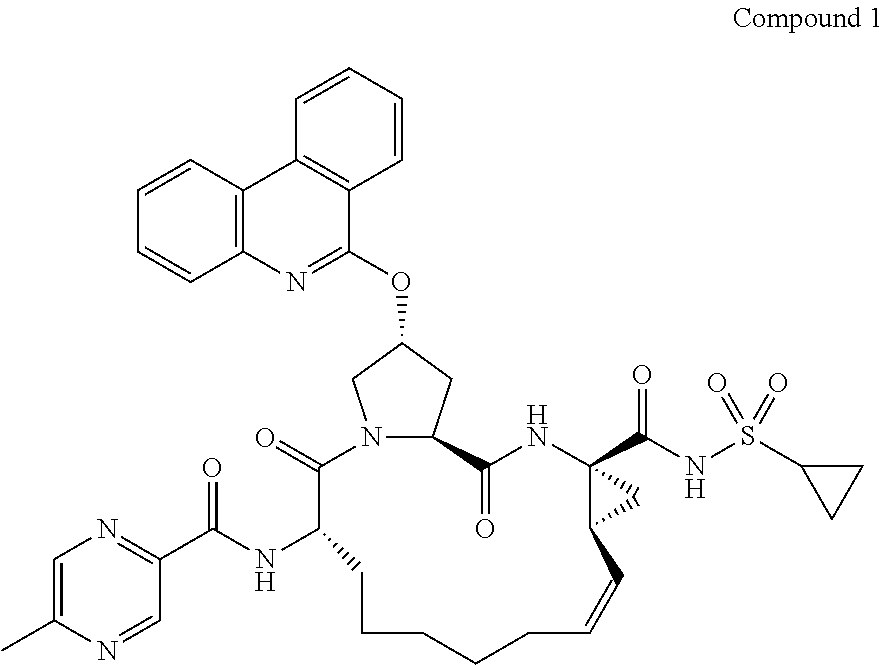
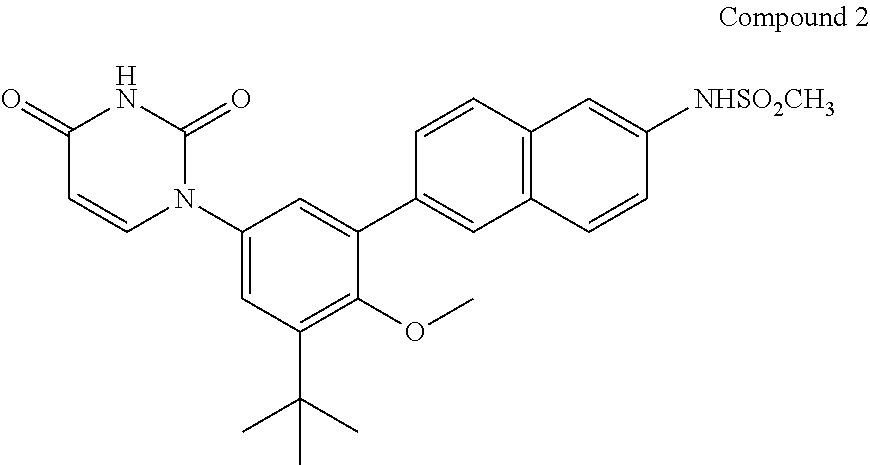
The present invention features interferon- and ribavirin-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents without interferon and ribavirin to a subject with HCV infection, wherein the treatment lasts for 12 weeks, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
Methods for Treating HCV
ActiveUS20130102558A1Avoid side effectsImprove pharmacokineticsBiocideDipeptide ingredientsCytochrome P450 InhibitorsShort duration
The present invention features interferon- and ribavirin-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents without interferon and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to a subject an effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), and an inhibitor of cytochrome P450 (e.g., ritonavir).
Owner:ABBVIE INC
Combination of two antivirals for treating hepatitis C
The present invention features interferon- and ribavirin-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents without interferon and ribavirin to a subject with HCV infection, wherein the treatment lasts for 12 weeks, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
Methods for Treating HCV
InactiveUS20150024999A1Improve pharmacokineticsImprove bioavailabilityOrganic active ingredientsBiocideCytochrome P450 InhibitorsShort duration
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to the subject effective amounts of therapeutic agent 1, therapeutic agent 2 (or therapeutic agent 3), an inhibitor of cytochrome P450 (e.g., ritonavir), and ribavirin.
Owner:ABBVIE INC
Combination of direct acting antiviral agents and ribavirin for treating HCV patients
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of interferon, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
Once daily treatment of hepatitis c with ribavirin and taribavirin
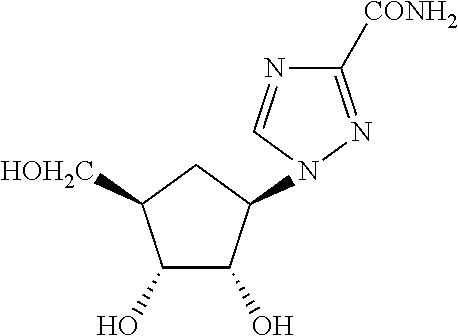
Hepatitis C is treated by administering once daily ribavirin, taribavirin, other derivatives or pharmaceutically acceptable salts thereof. Hepatitis C may also be treated by administering any of the foregoing compounds once daily in combination with interferon and / or direct-acting antivirals. Once daily dosage forms administered for treating hepatitis C may comprise between 800 mg and 1400 of ribavirin. Once daily dosage forms administered for treating hepatitis C may also comprise between 800 mg and 4000 mg of taribavirin.
Owner:ABBVIE INC
Methods for treating hcv
InactiveUS20140274934A1Avoid side effectsEfficient managementBiocideDigestive systemShort durationPharmacology
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of interferon, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
Methods for Treating HCV
ActiveUS20150283198A1Avoid side effectsEfficient managementBiocideOrganic active ingredientsShort durationInterferon alpha
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of either interferon or ribavirin, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
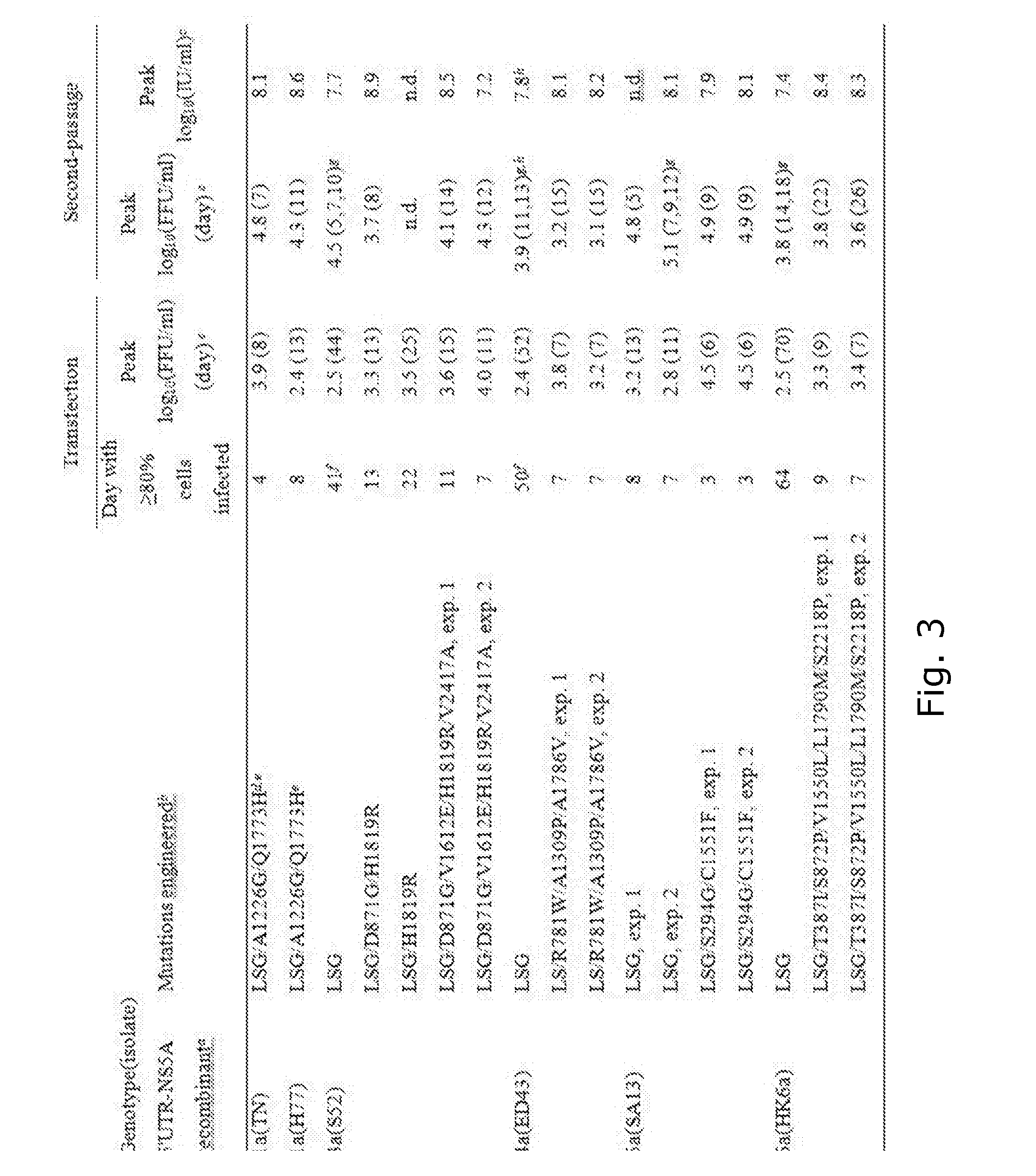
Novel hcv culture systems and direct-acting antiviral sensitivity
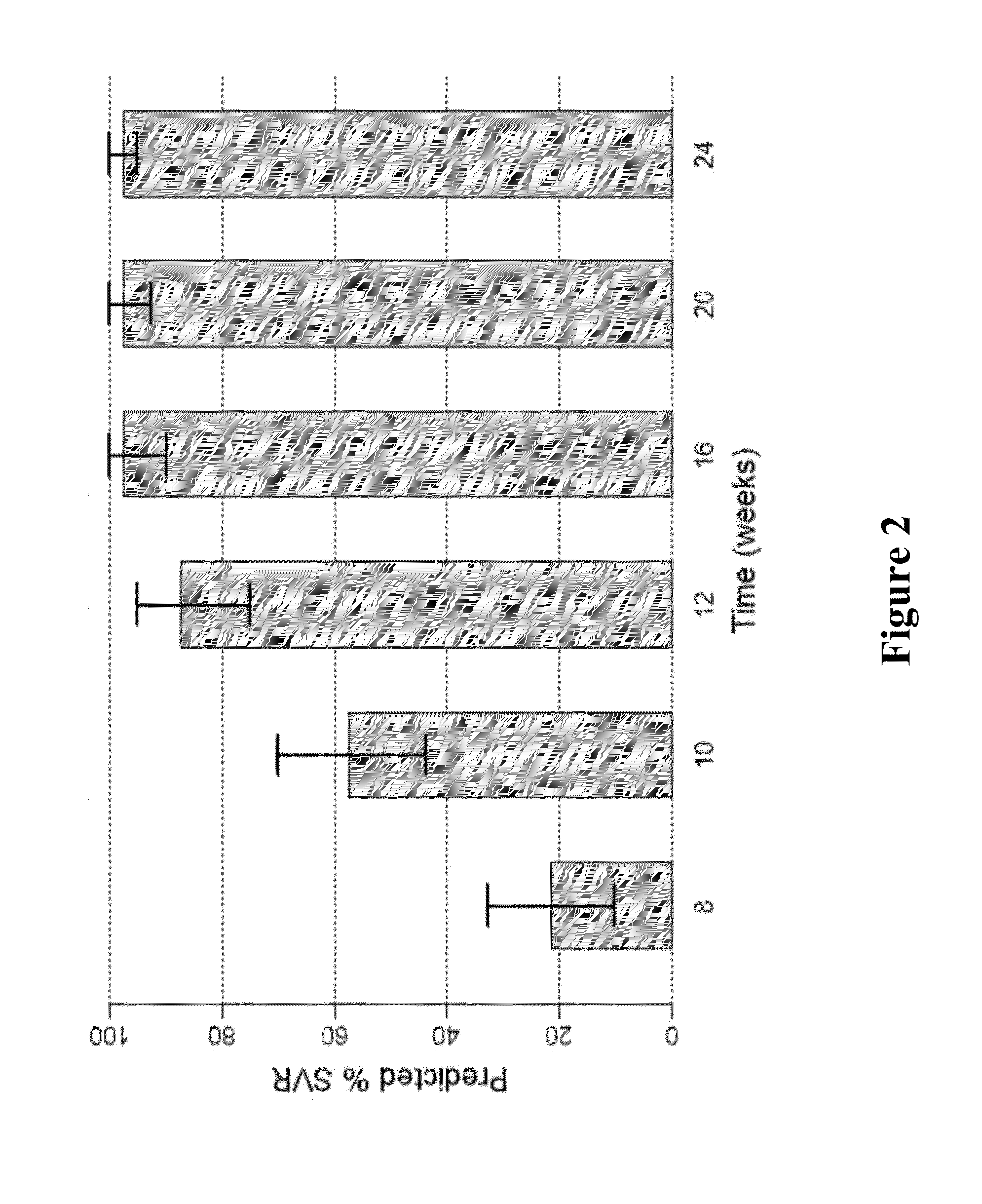
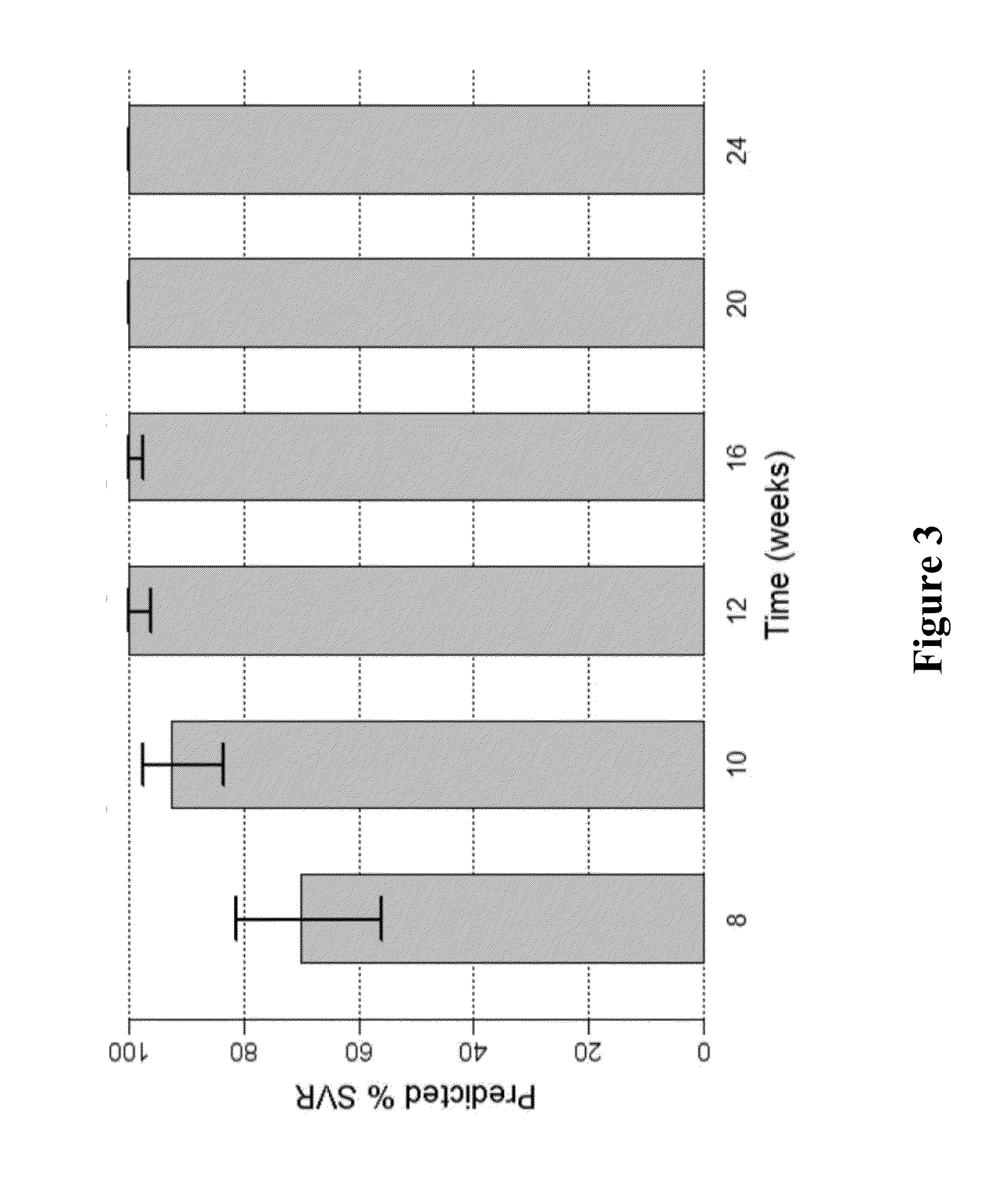
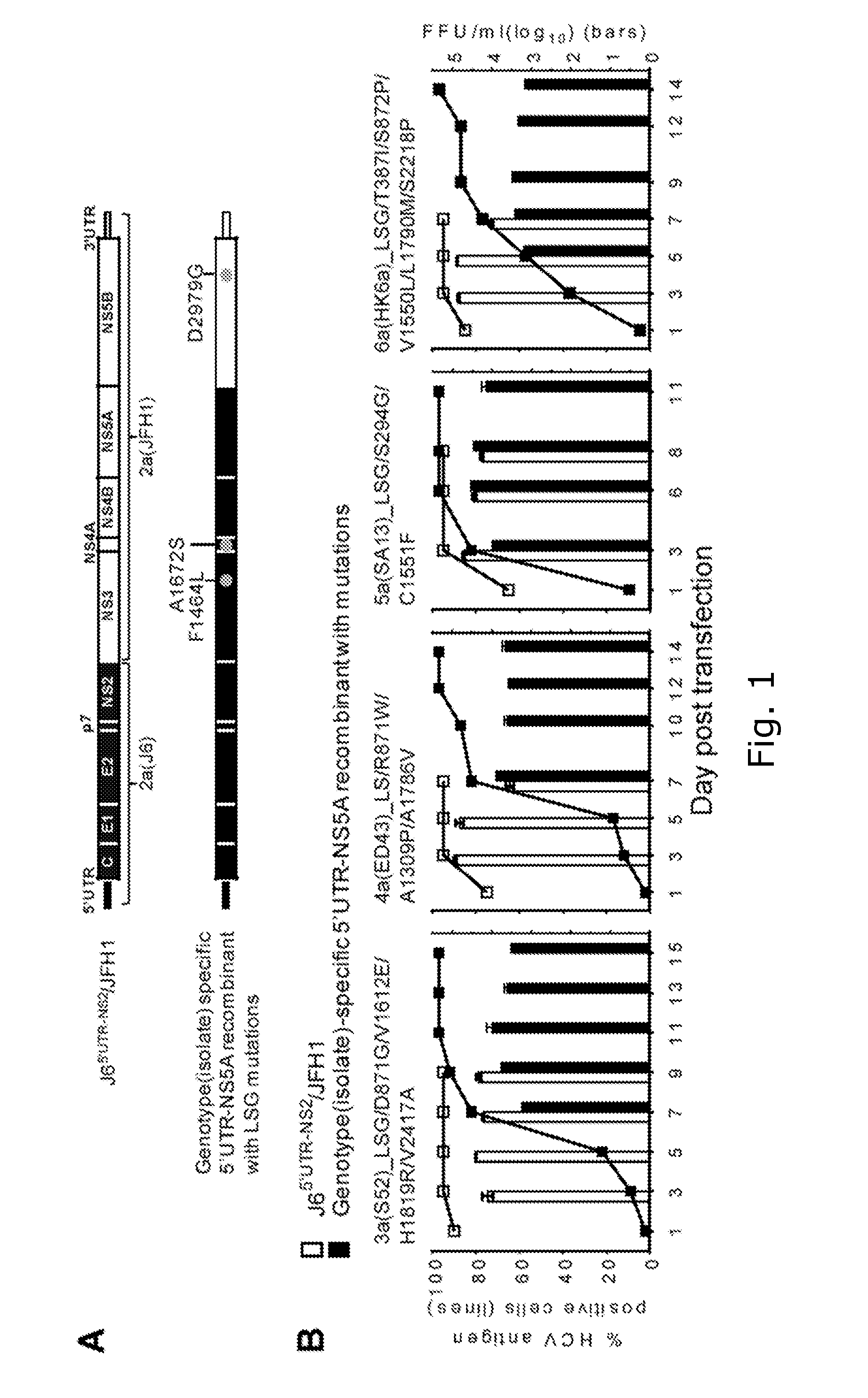
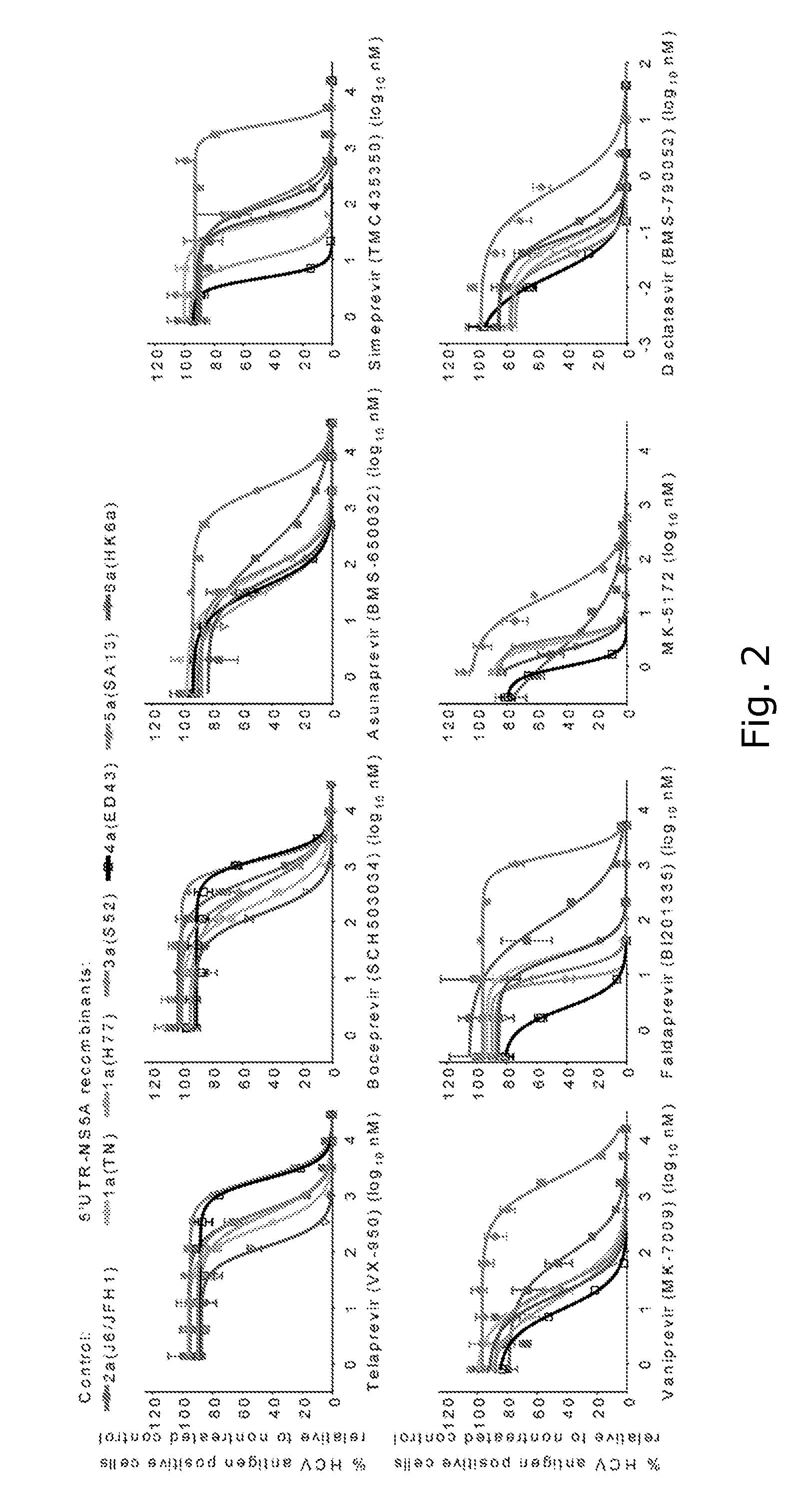
ActiveUS20160244729A1SsRNA viruses positive-senseMicrobiological testing/measurementIndividualized treatmentBasic research
The present invention relates to hepatitis C virus (HCV) culture systems of genotypes 1a, 3a, 4a, 5a, and 6a that directly contribute to HCV drug and vaccine development, to HCV basic research and better-individualized treatment of HCV infected patients.
Owner:HVIDOVRE HOSPITAL
Methods for Treating HCV
PendingUS20170333428A1Avoid side effectsEfficient managementOrganic active ingredientsMedicineShort duration
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of either interferon or ribavirin, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
Methods for Treating HCV
InactiveUS20170360783A1Avoid side effectsEfficient managementOrganic active ingredientsShort durationInterferon alpha
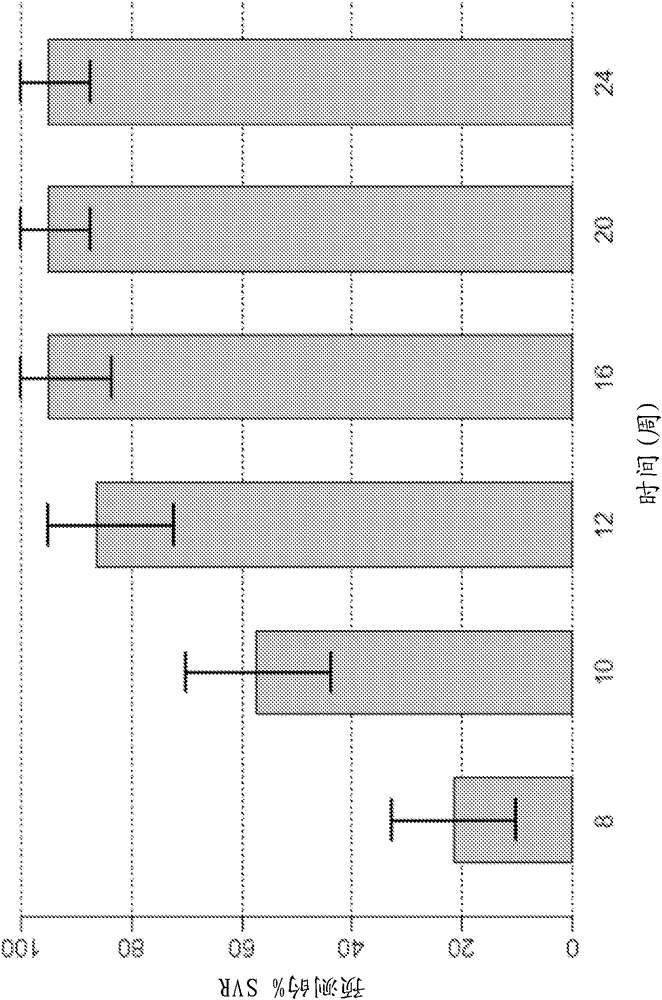
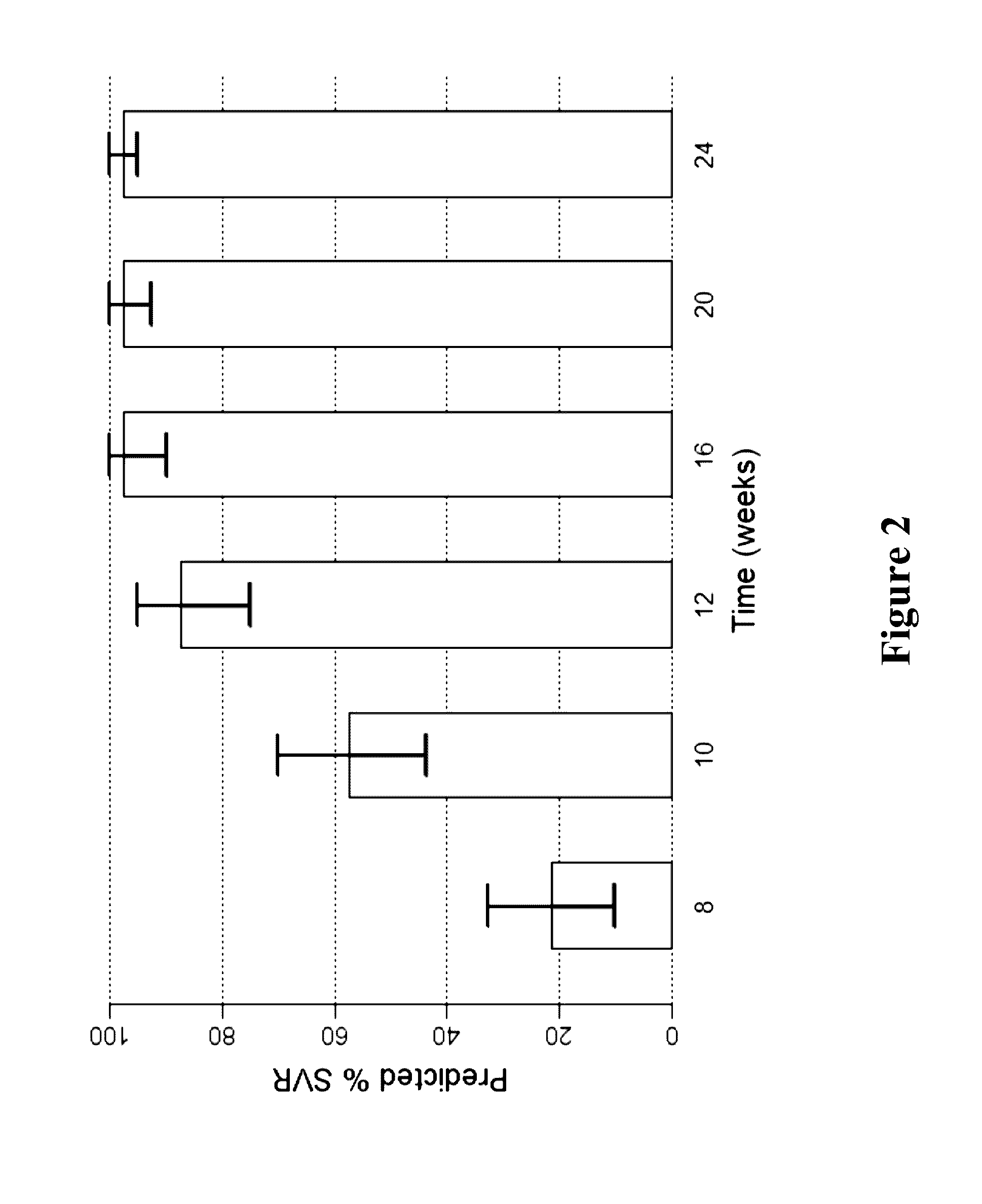
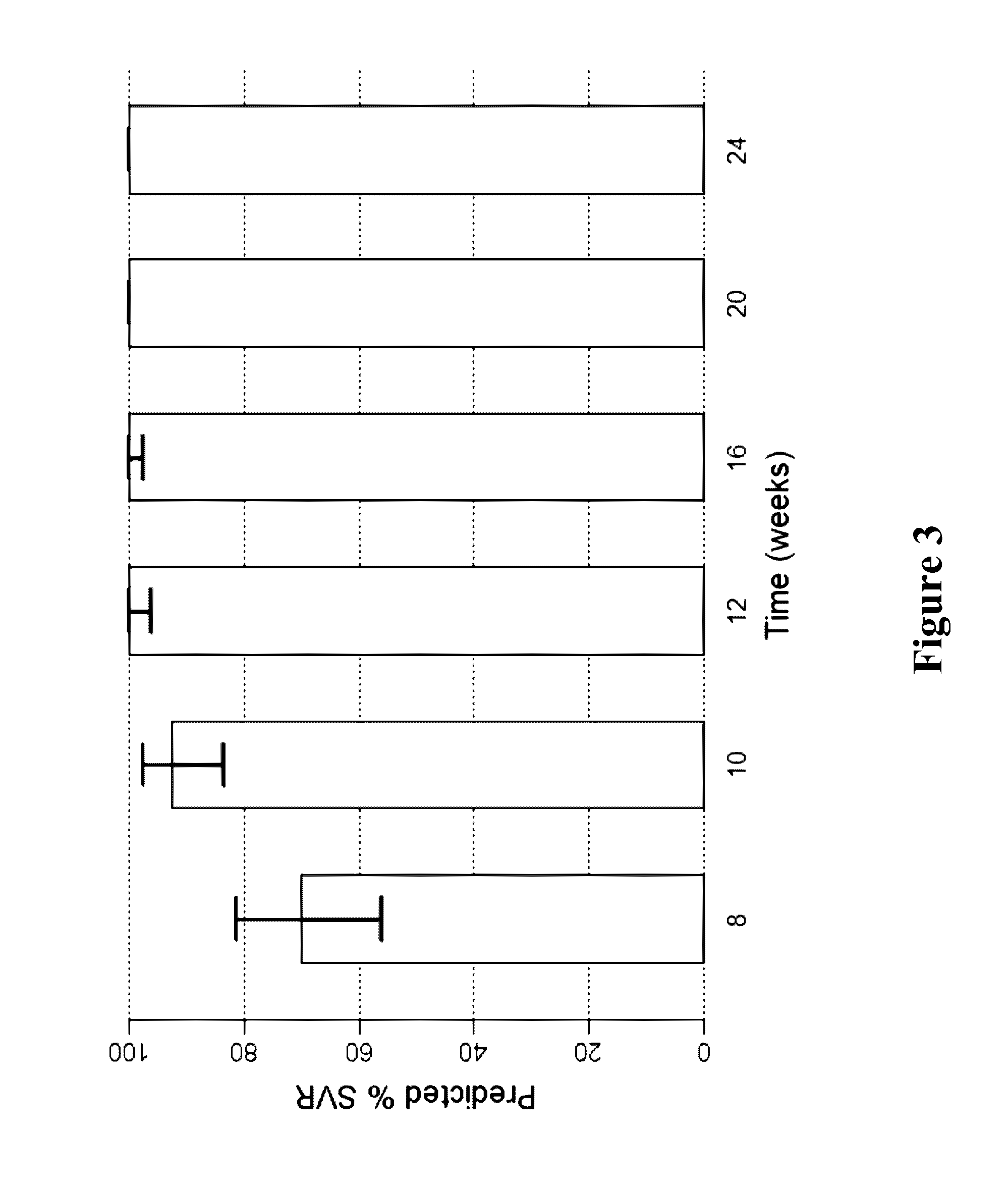
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 16 weeks, alternatively no more than 12 weeks, or alternatively no more than 8 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents to a subject with HCV infection, wherein the treatment lasts for 16, 12, or 8 weeks and does not include administration of either interferon or ribavirin, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
Methods for Treating HCV
ActiveUS20160317602A9Organic active ingredientsDipeptide ingredientsChemical compoundPharmaceutical medicine
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of either interferon or ribavirin, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
Methods for treating hcv
InactiveUS20160228496A1Improve pharmacokineticsImprove bioavailabilityOrganic active ingredientsDipeptide ingredientsMedicineCytochrome P450 Inhibitors
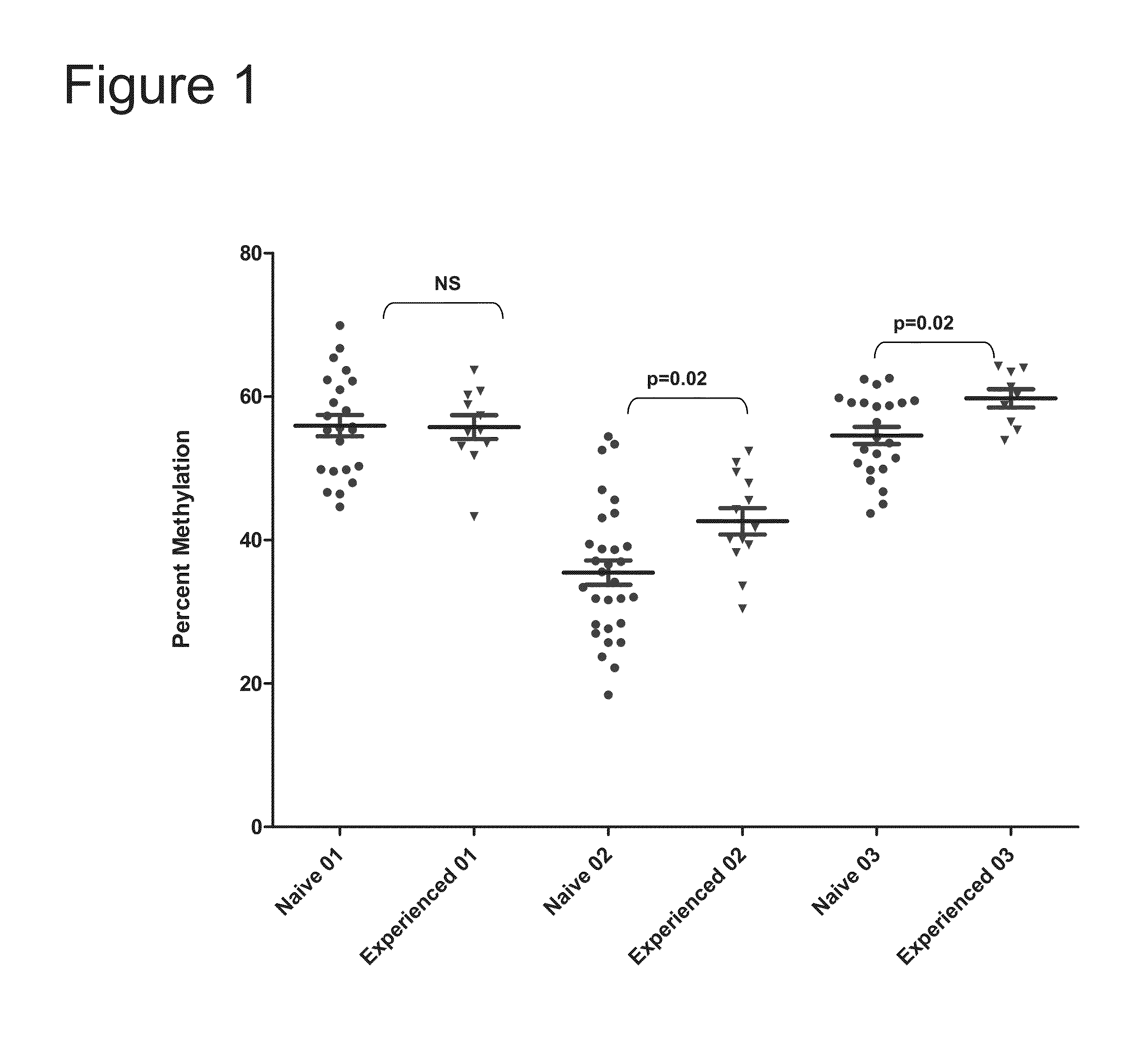
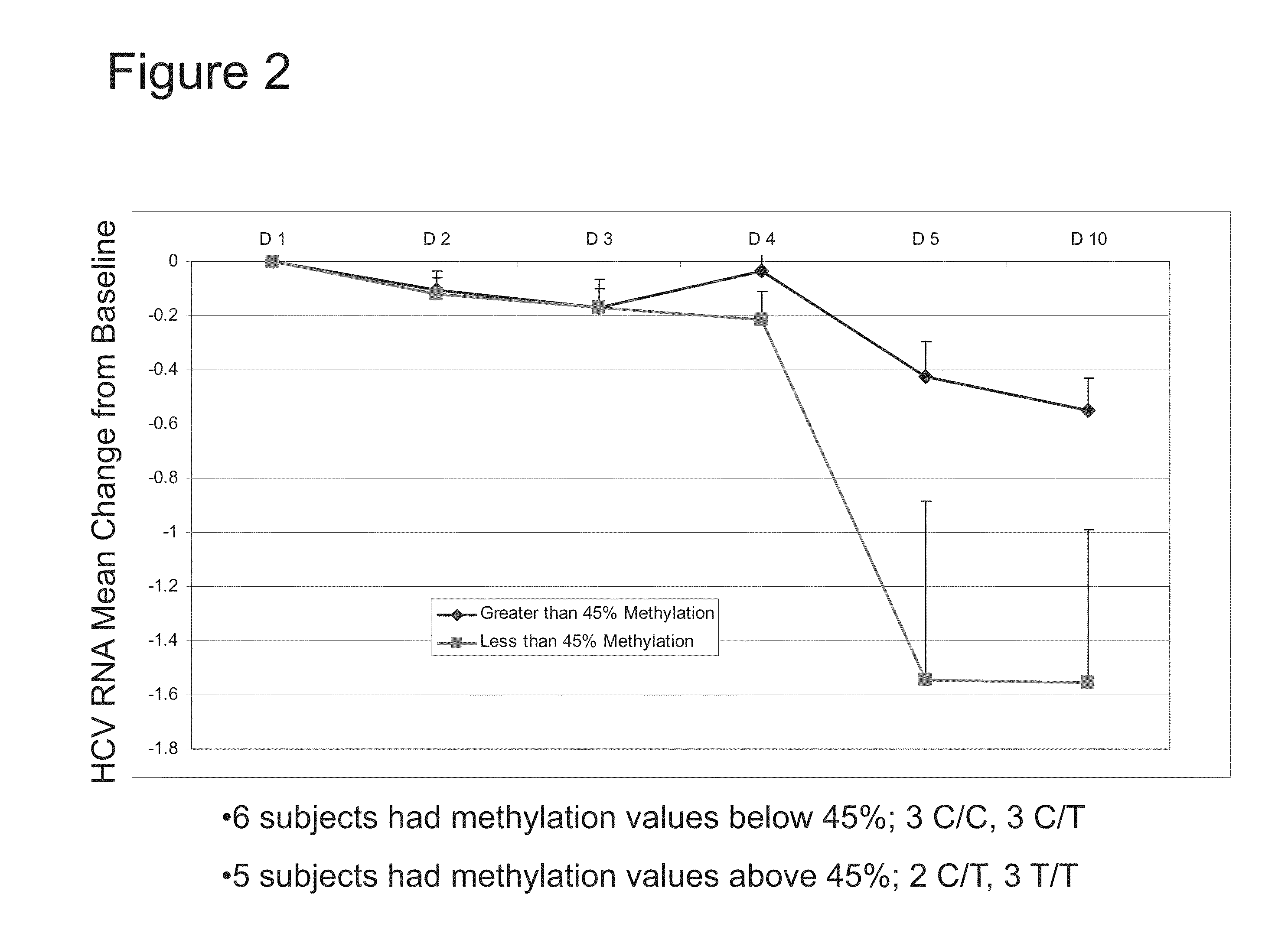
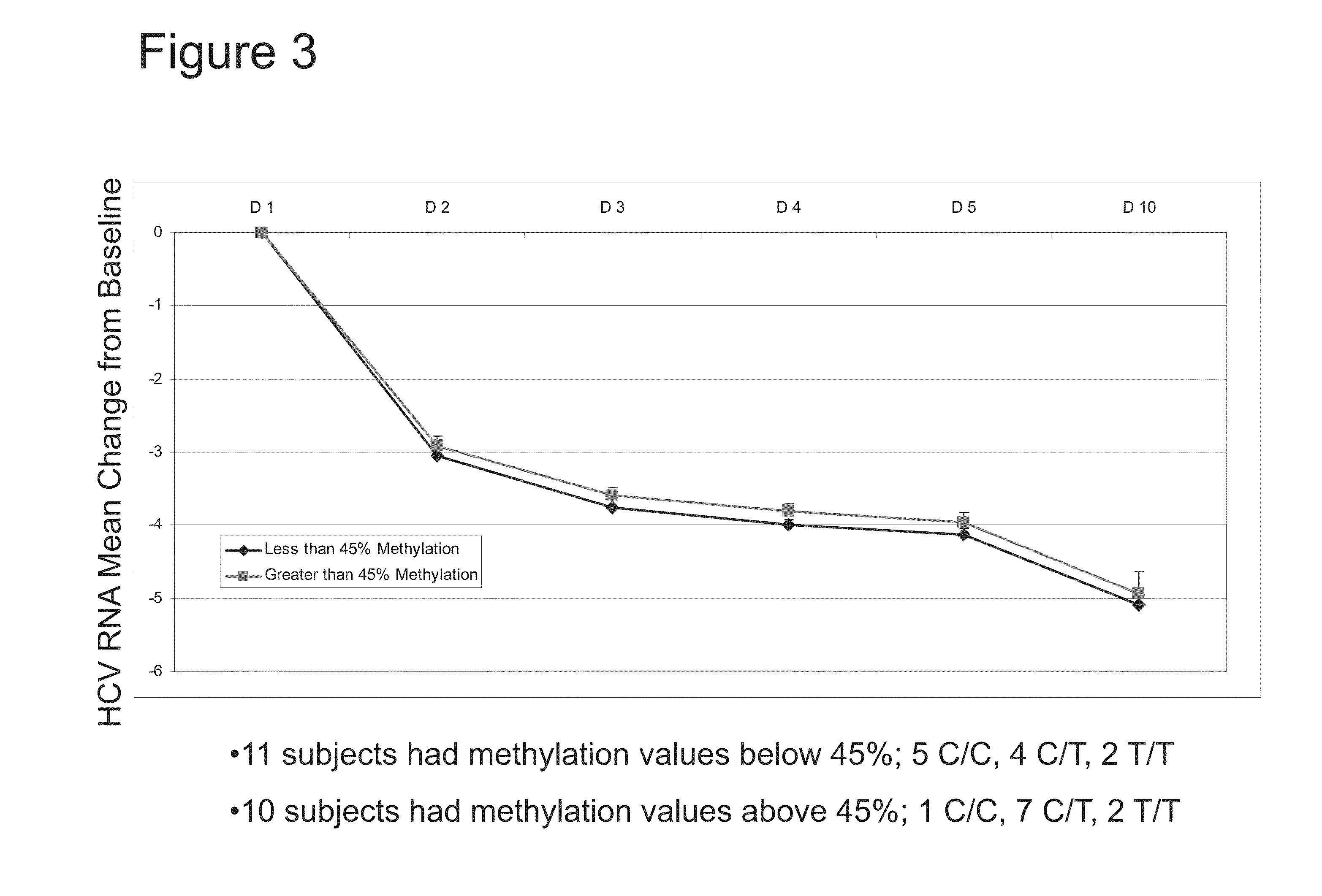
The present invention features therapies for the treatment of HCV comprising direct-acting antiviral agents. Preferably, the treatment is administered to an HCV-infected patient who has been tested to determine methylation status of a CpG island within a promoter region of the IL28B gene. In one aspect, the therapies comprise administering one or more direct acting antiviral agents and, optionally ribavirin, to a subject with HCV infection. For example, the therapies comprise administering to the subject effective amounts of therapeutic agent 1, therapeutic agent 2, therapeutic agent 3, an inhibitor of cytochrome P450 (e.g., ritonavir), and / or ribavirin.
Owner:ABBVIE INC
Methods for Treating HCV
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of interferon, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
Methods for Treating HCV
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of interferon, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
Methods for treating hcv
InactiveUS20160237491A1Improve pharmacokineticsImprove bioavailabilityOrganic active ingredientsDipeptide ingredientsInfected patientMedicine
The present invention features therapies for the treatment of HCV comprising direct-acting antiviral agents. Preferably, the treatment is administered to an HCV-infected patient who has been tested to determine methylation status of a CpG island within a promoter region of the IL28B gene. In one aspect, the therapies comprise administering one or more direct acting antiviral agents and, optionally ribavirin, to a subject with HCV infection. For example, the therapies comprise administering to the subject effective amounts of therapeutic agent 1, therapeutic agent 2, therapeutic agent 3, an inhibitor of cytochrome P450 (e.g., ritonavir), and / or ribavirin.
Owner:ABBVIE INC
Methods for Treating HCV
InactiveUS20180177779A1Avoid side effectsEfficient managementOrganic active ingredientsAntiviralsShort durationInterferon alpha
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of either interferon or ribavirin, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
Methods for treating hcv
InactiveUS20160333404A1Improve pharmacokineticsImprove bioavailabilityOrganic active ingredientsPeptide/protein ingredientsMedicineCytochrome P450
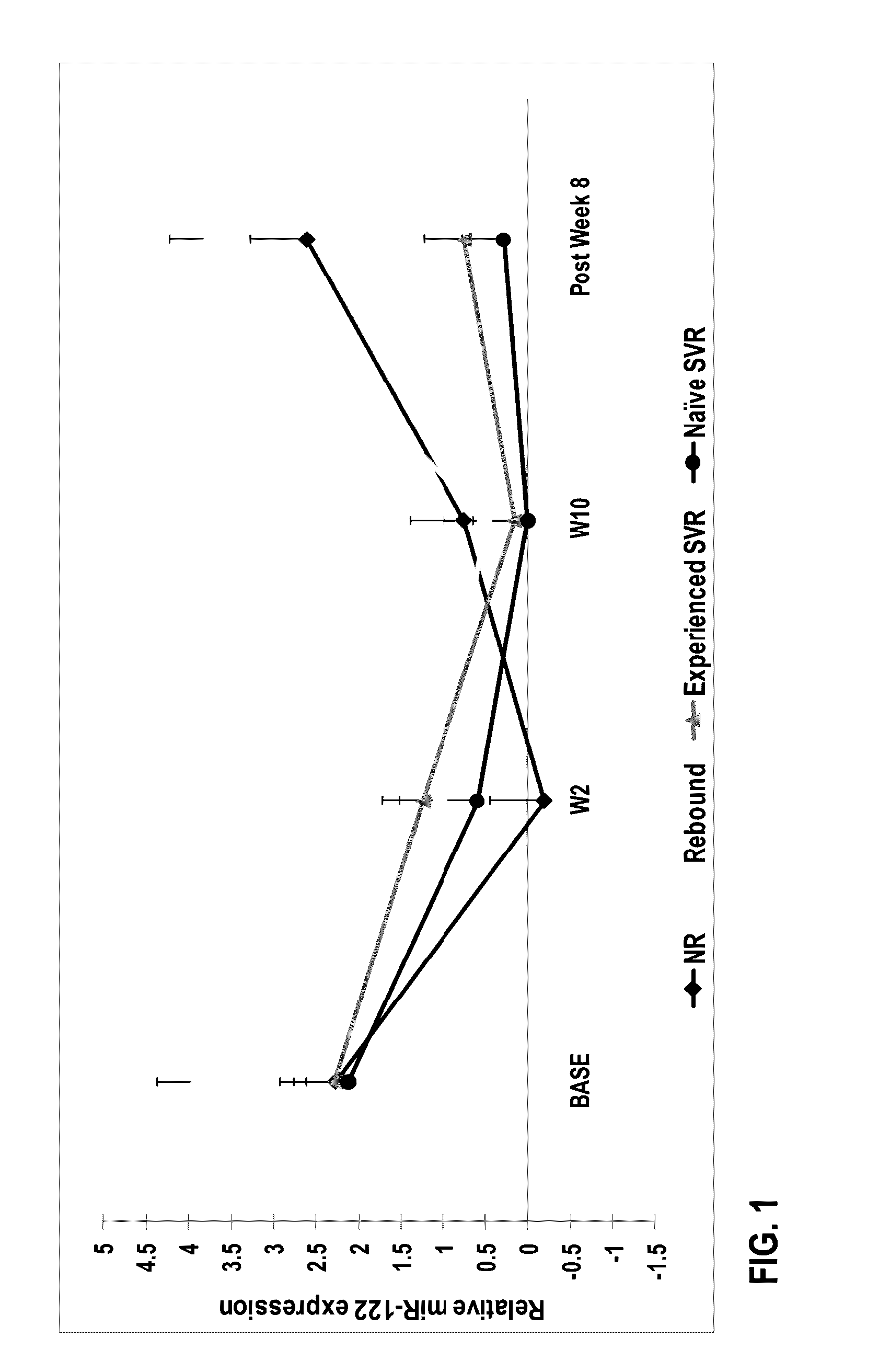
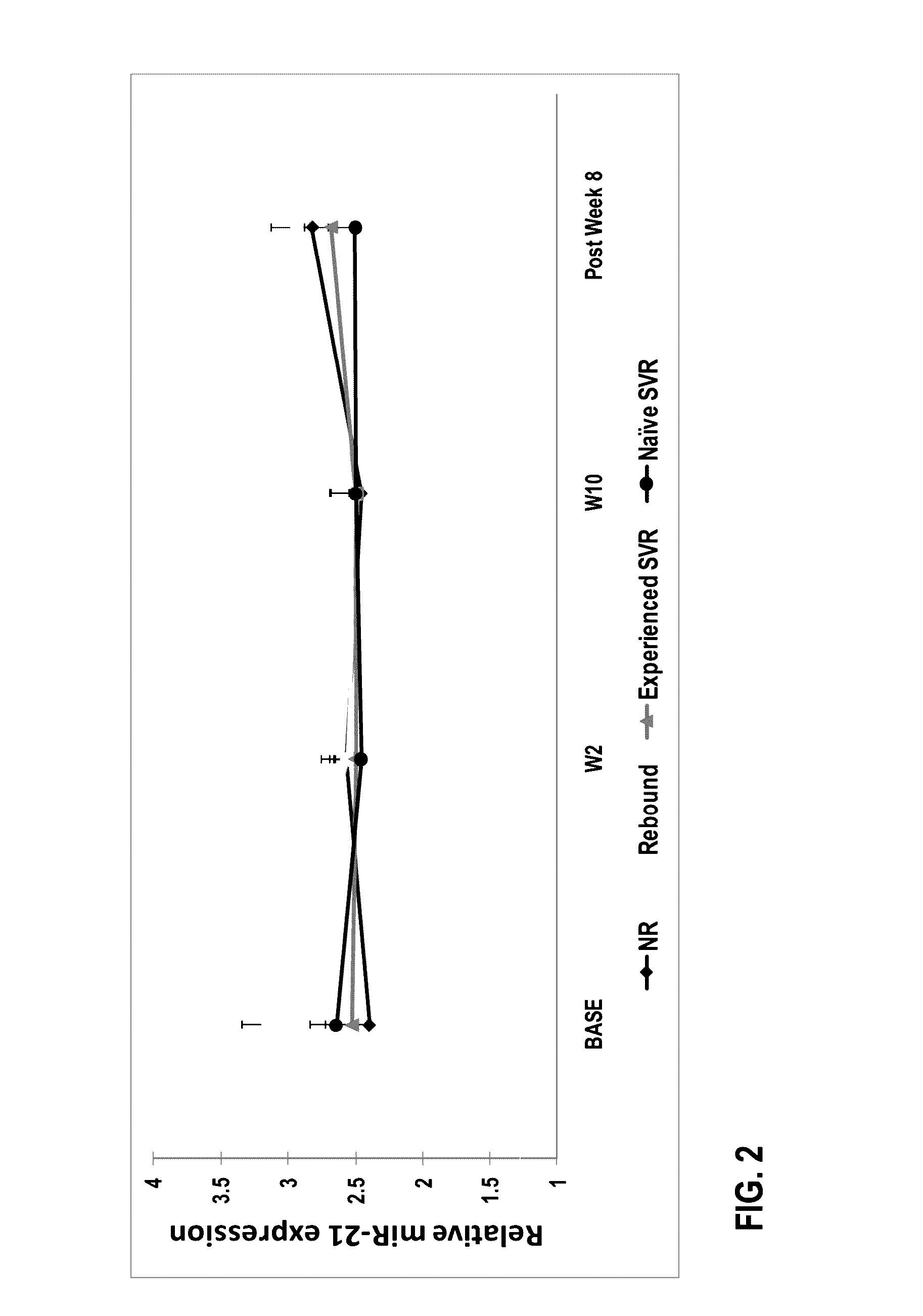
The present invention features therapies for the treatment of HCV comprising direct-acting antiviral agents. Preferably, the treatment is administered to an HCV-infected patient who has been tested to determine expression levels of microRNAs such as miR-122 or miR-21. In one aspect, the therapies comprise administering one or more direct acting antiviral agents and, optionally ribavirin, to a subject with HCV infection. For example, the therapies comprise administering to the subject effective amounts of therapeutic agent 1, therapeutic agent 2, an inhibitor of cytochrome P450 (e.g., ritonavir), and ribavirin.
Owner:ABBVIE INC
Methods for treating HcV
Owner:ABBVIE INC
Methods for Treating HCV
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, thetreatment comprises administering at least two direct acting antiviral agents to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of either interferon or ribavirin, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
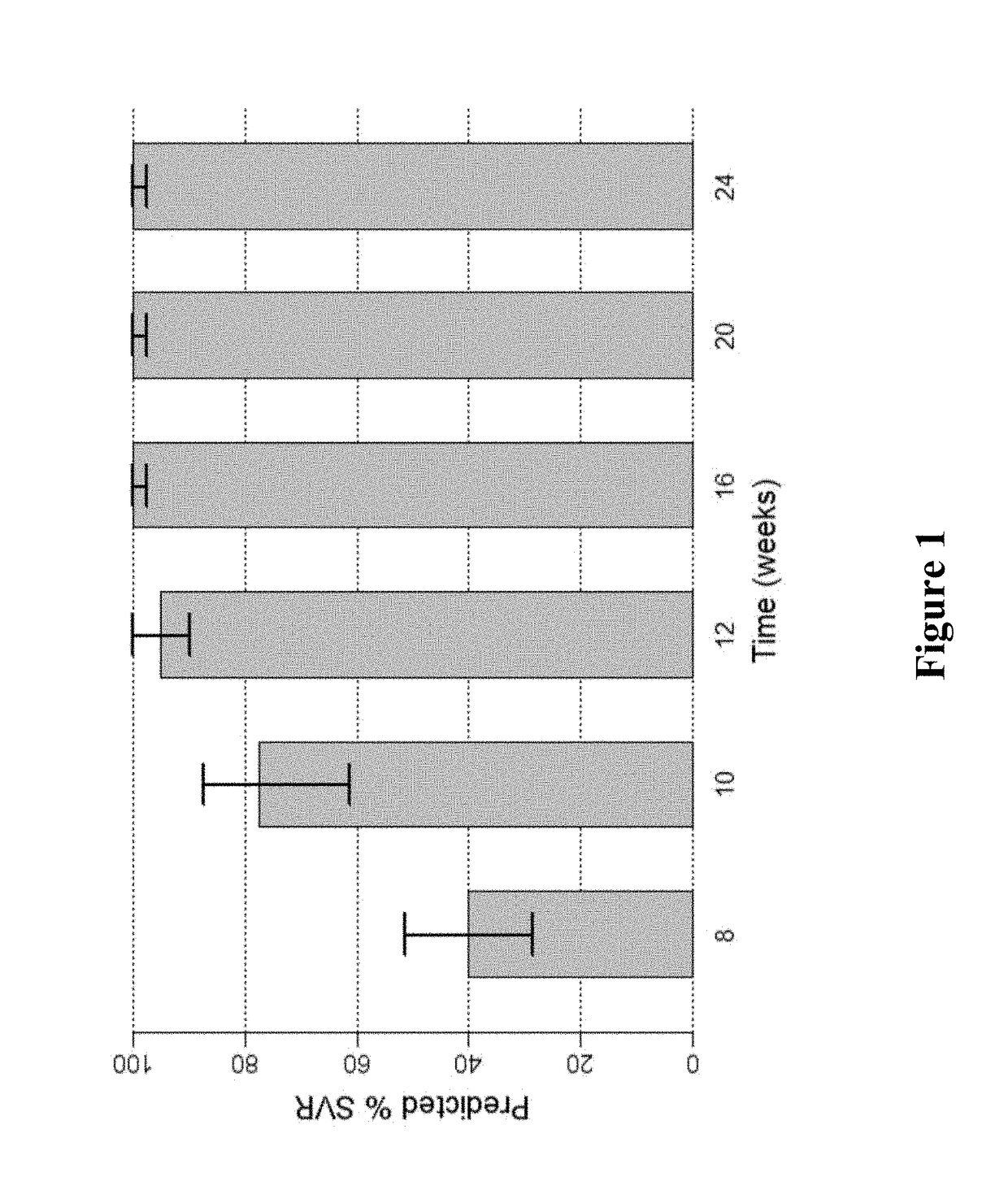
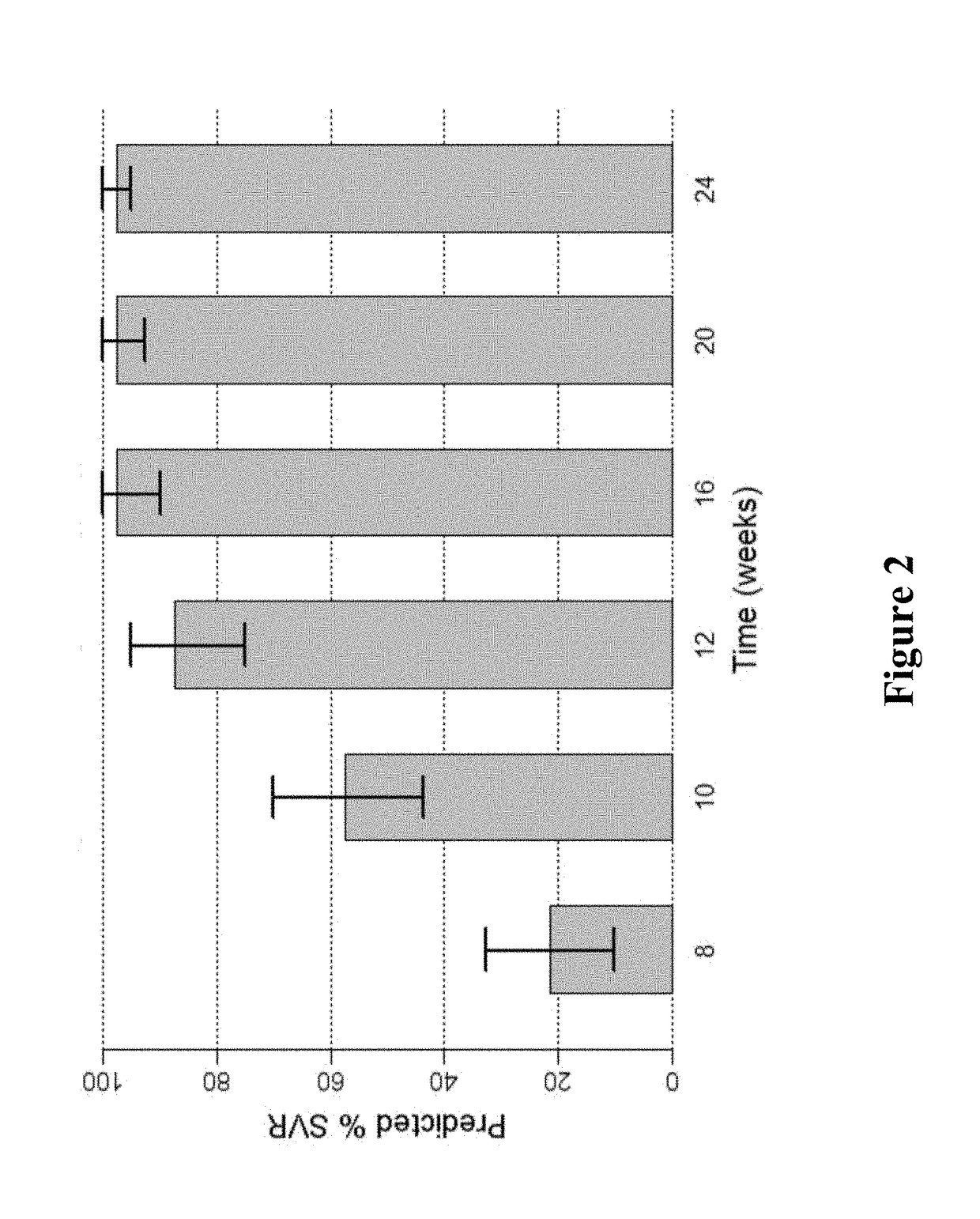
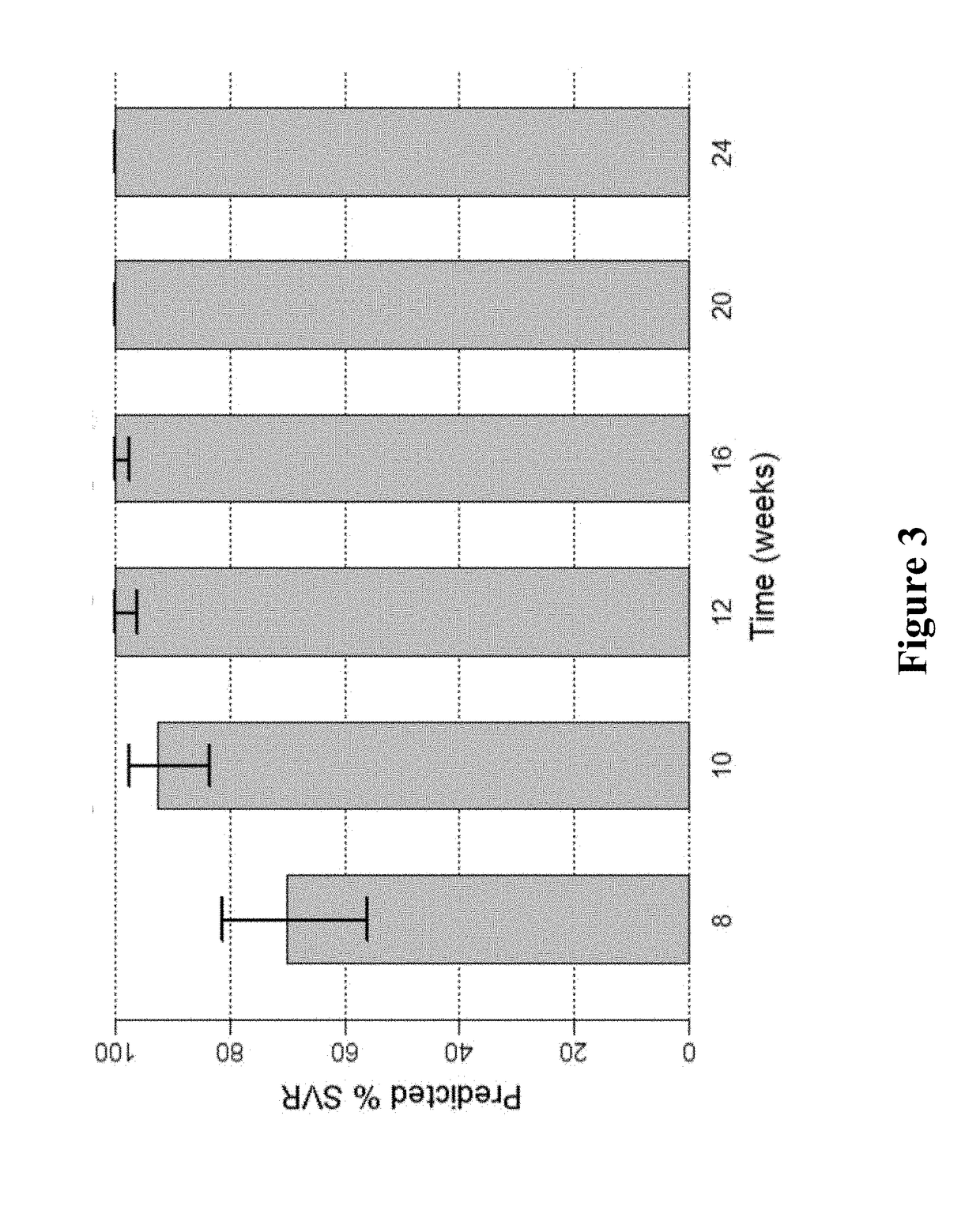
Response-guided hcv therapy
InactiveUS20180311268A1Clear the virus more effectivelyOrganic active ingredientsAntiviralsTolerabilityRegimen
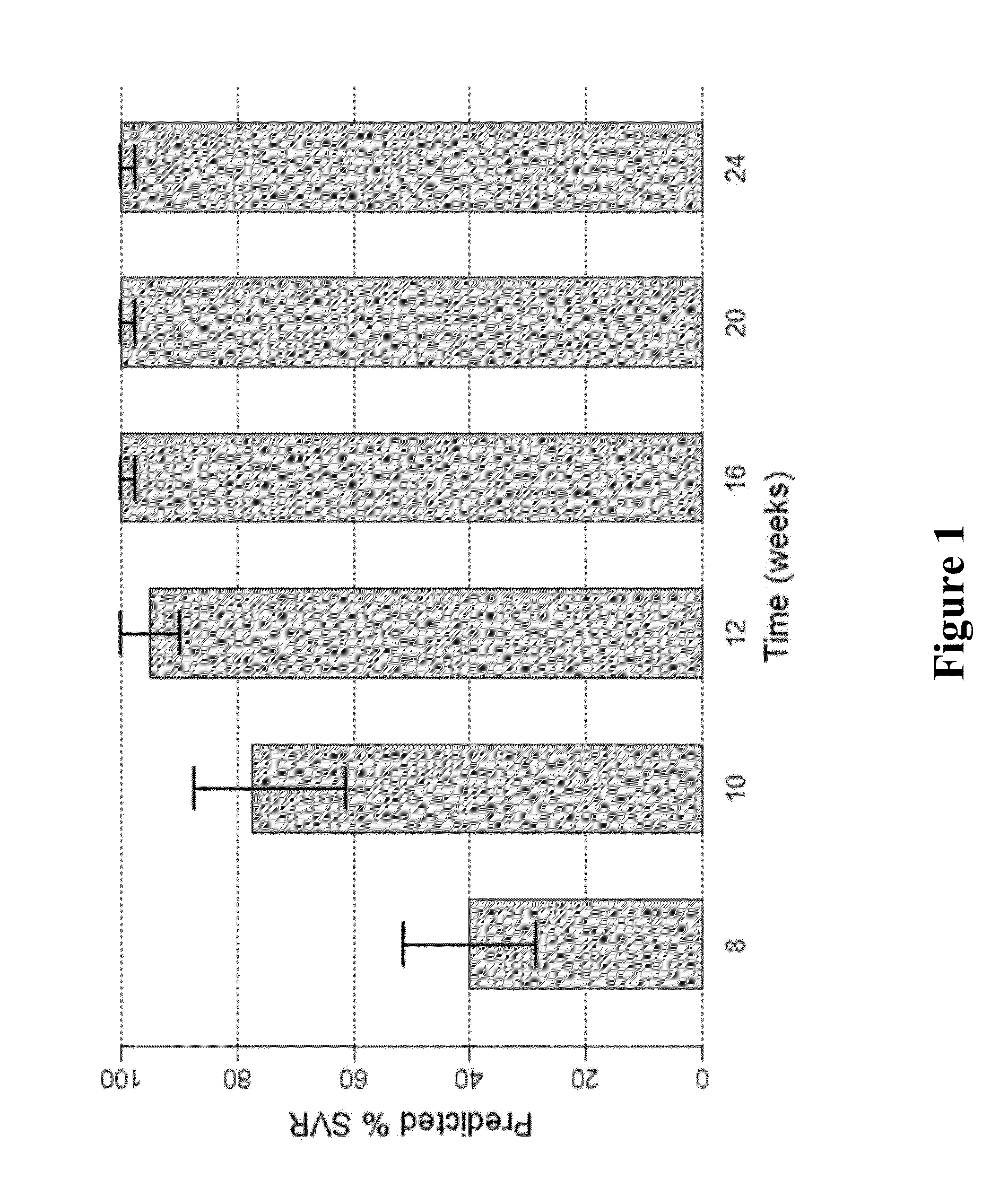
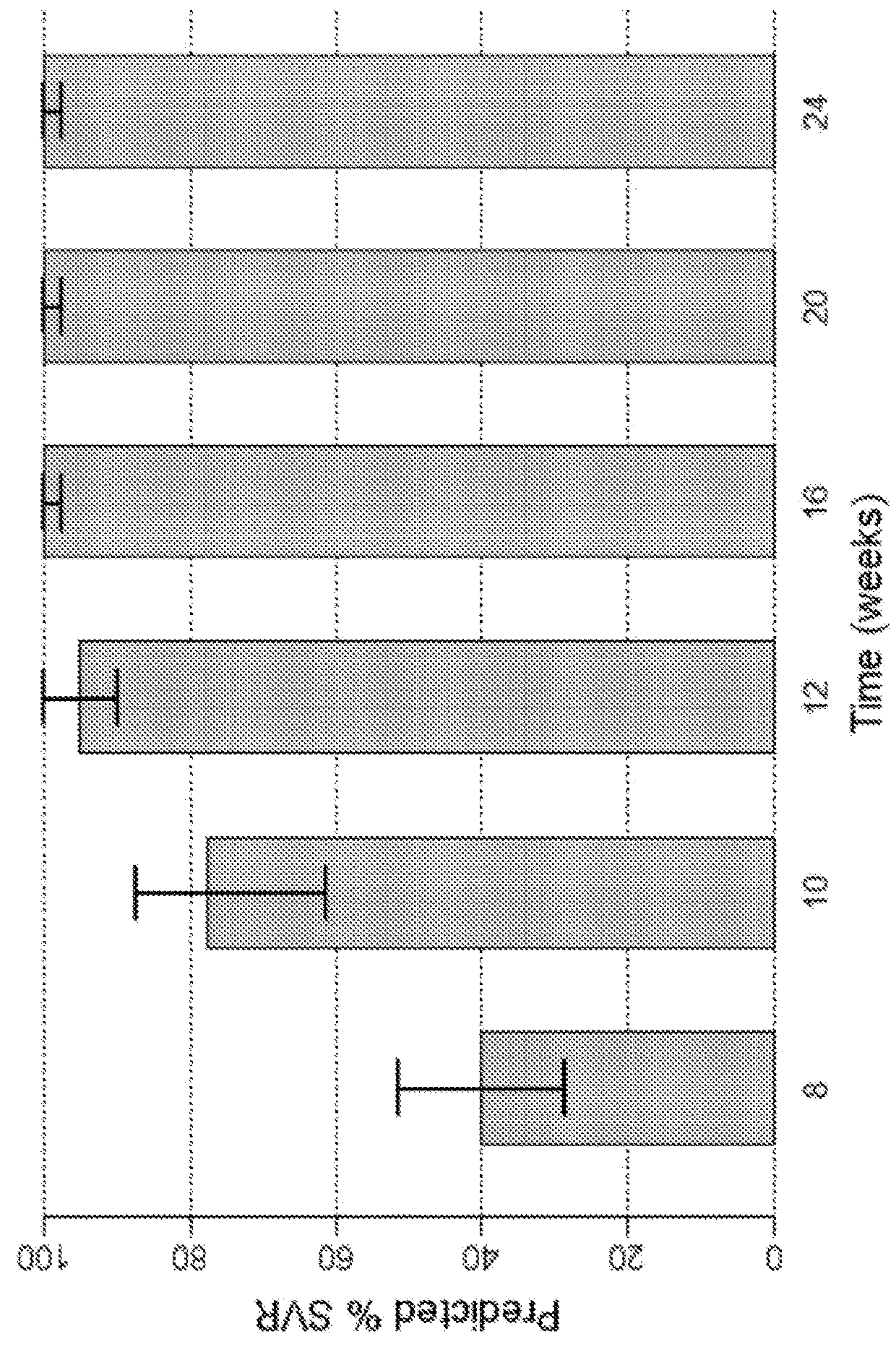
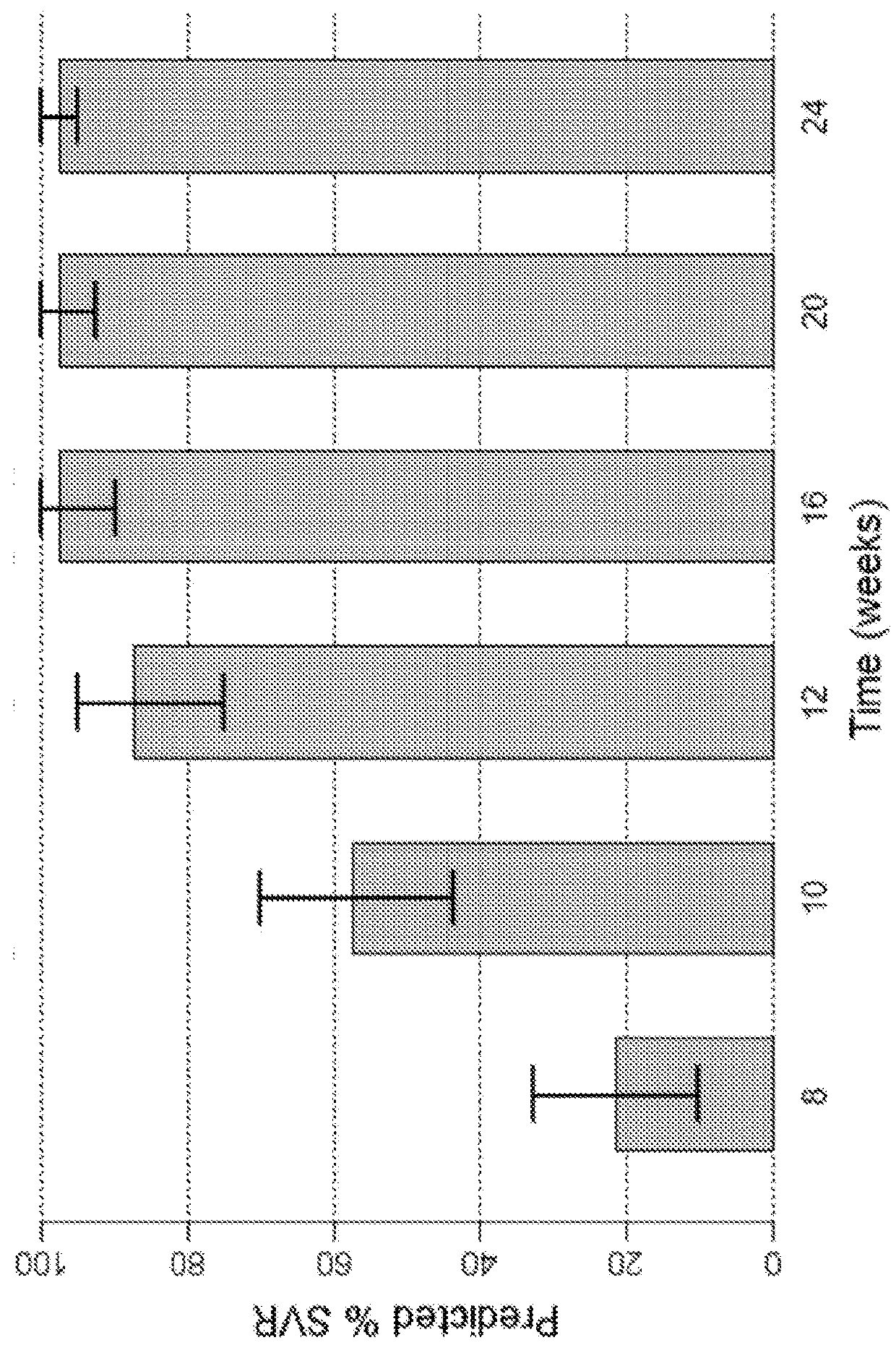
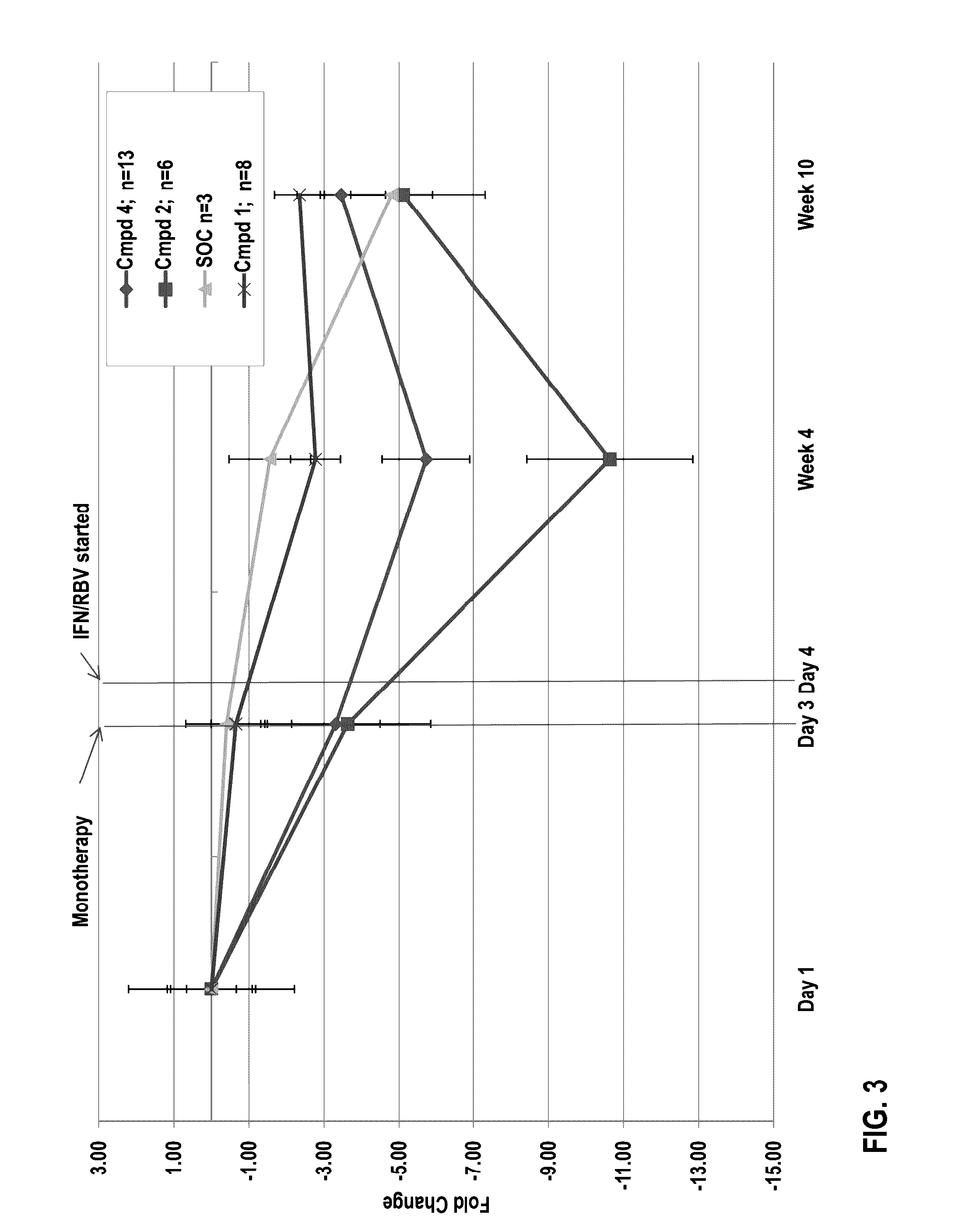
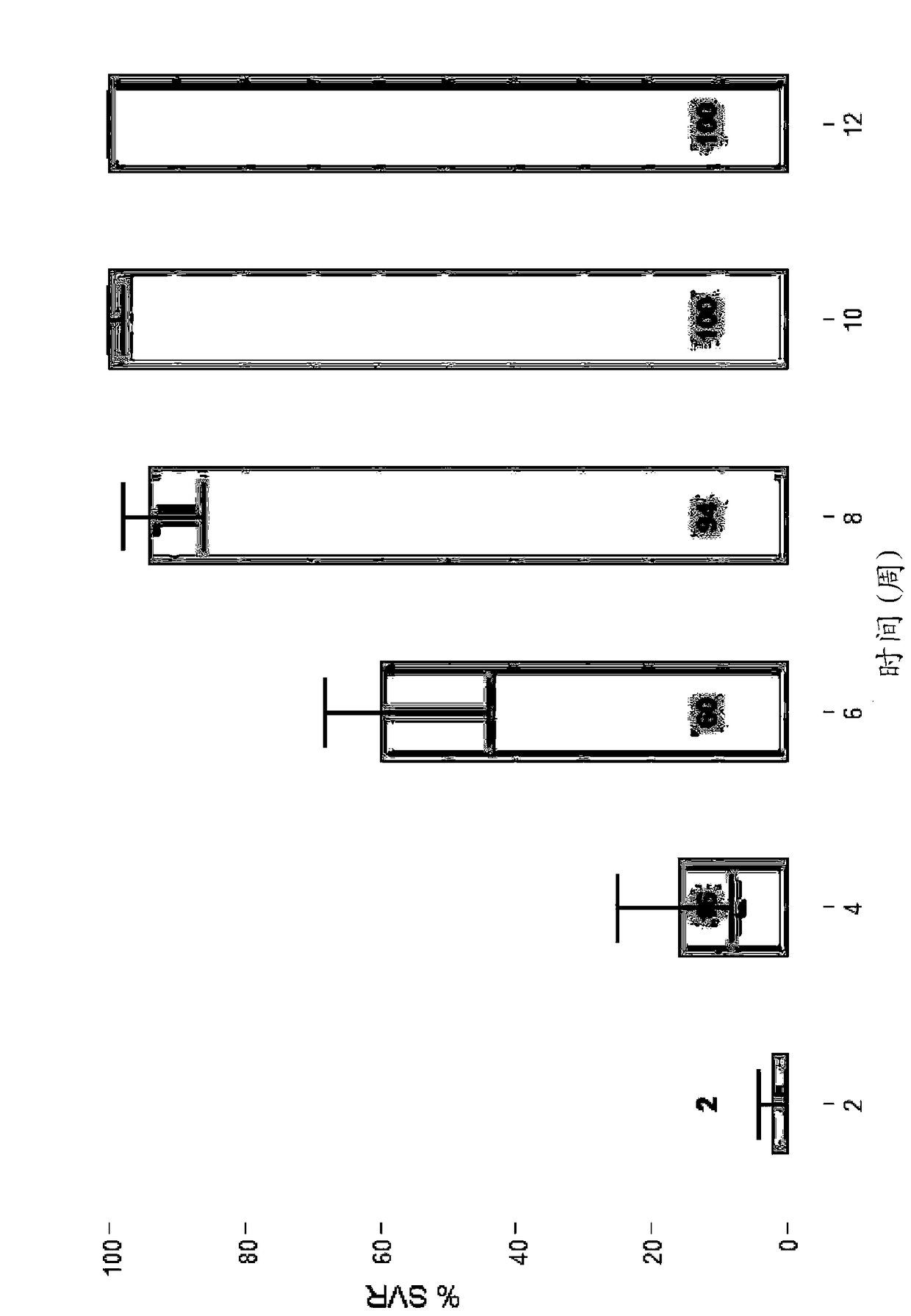
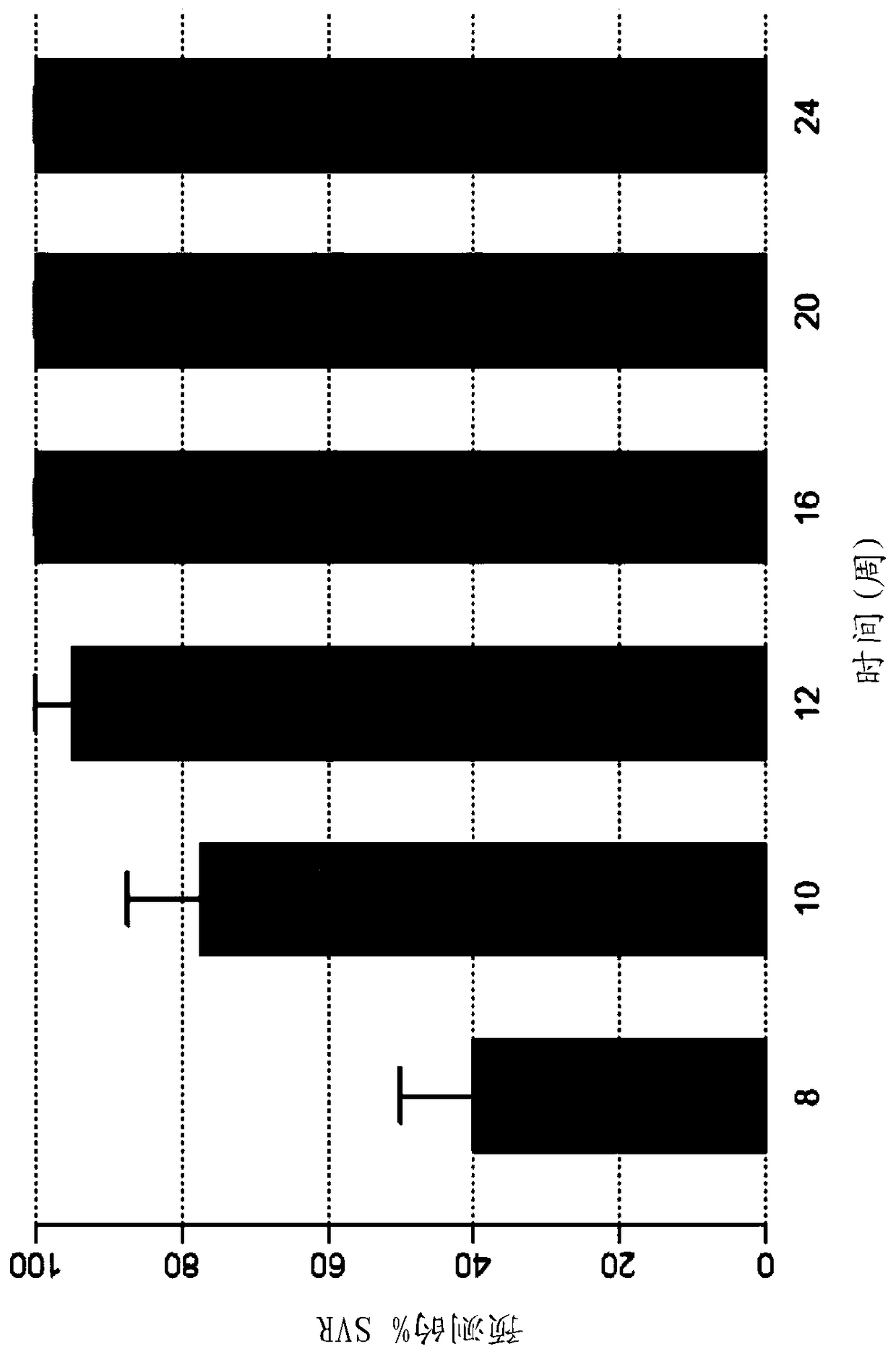
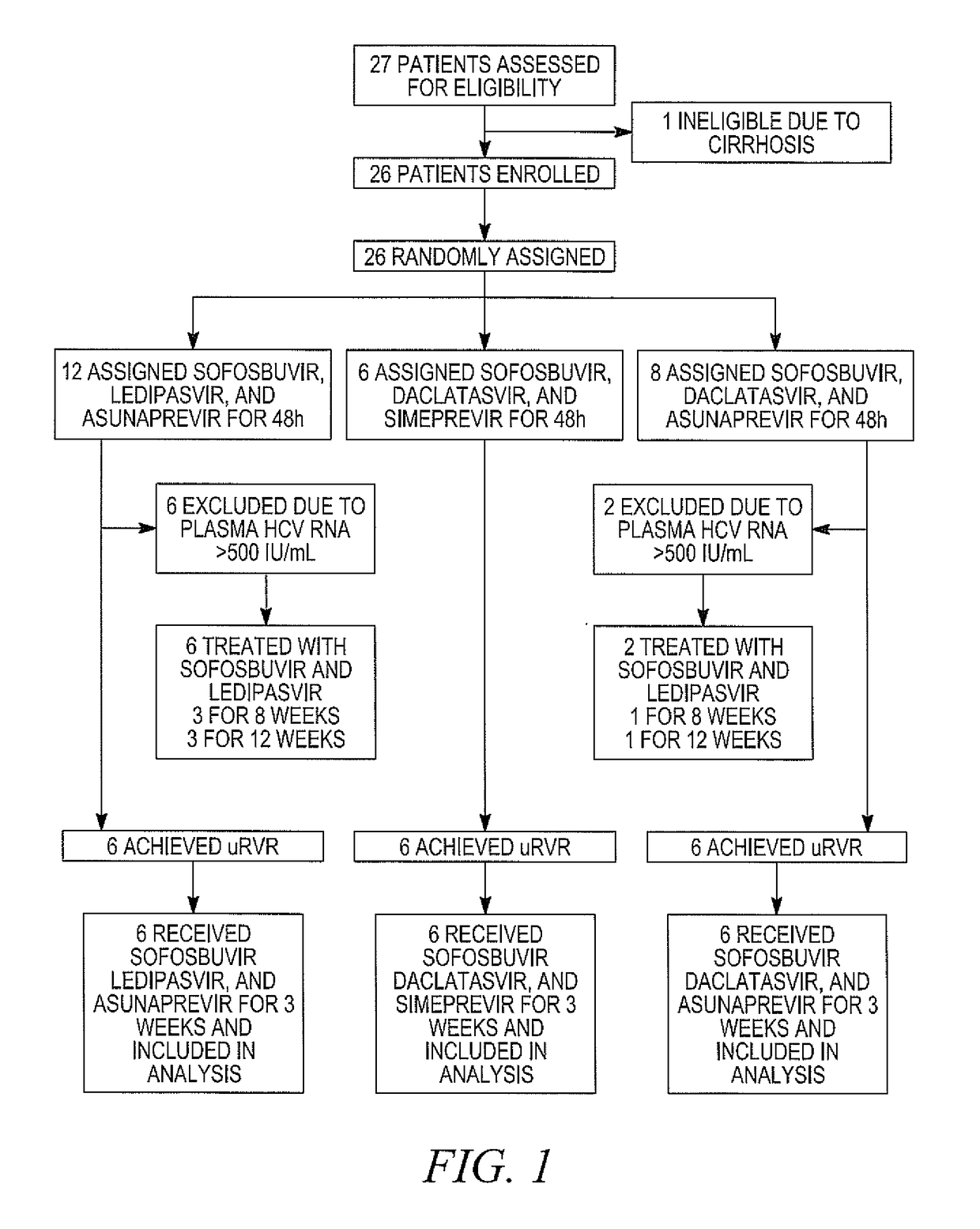
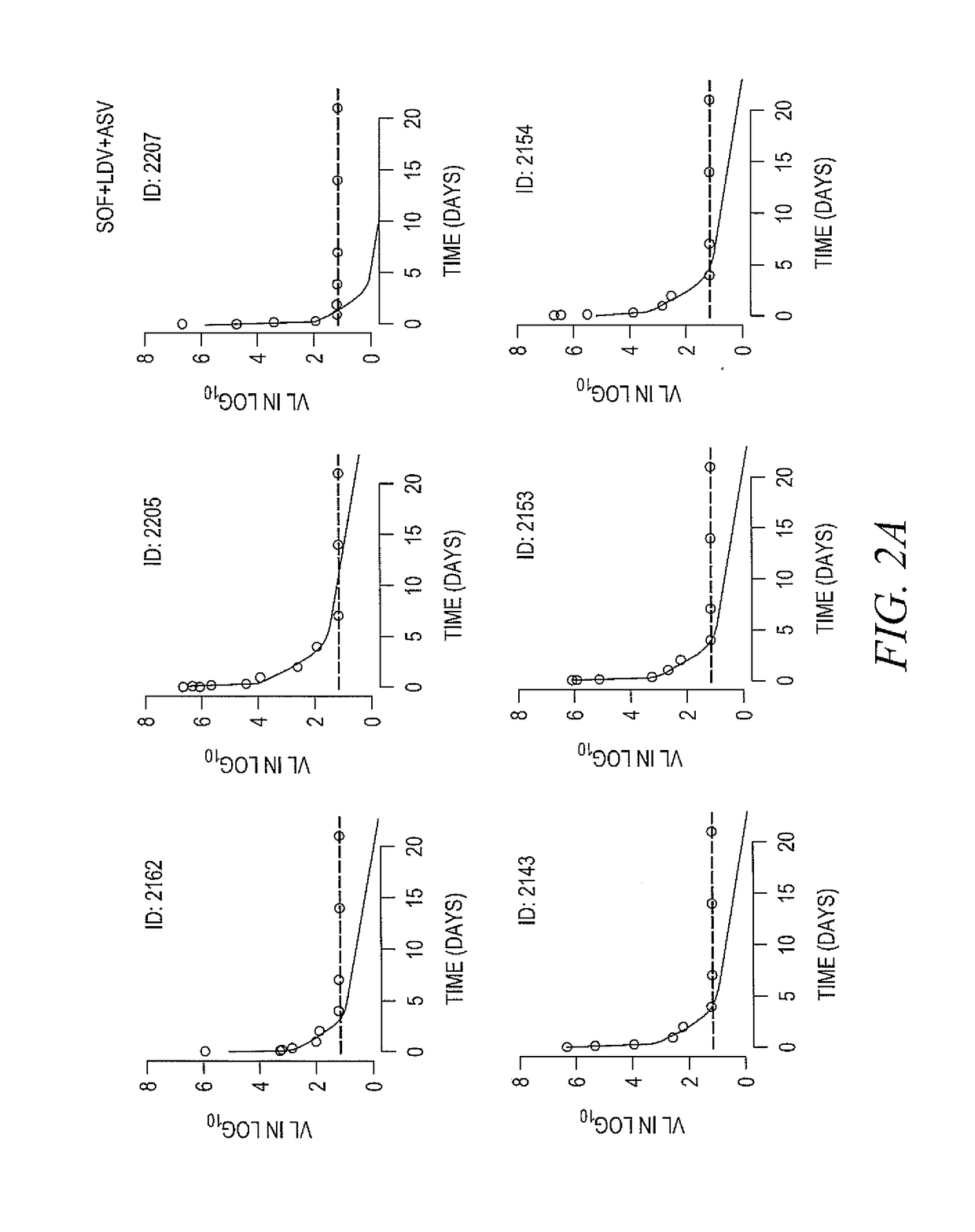
The present disclosure relates to solid dosage forms comprising anti-HCV compounds and methods of using such dosage forms to treat or prevent HCV infection. Direct-acting antiviral agents (DAAs) have a high cure rate, and favorable tolerability in persons infected with hepatitis C virus (HCV). However, shorter courses of therapy can improve adherence, affordability, and increase DAAs accessibility. The addition of an NS3 protease inhibitor to dual NS5A-NS5B (nucleoside) inhibitors enhances antiviral efficacy, and reduces treatment duration to 3 weeks (wks) in individuals with a rapid virologic response (RVR), defined as plasma HCV RNA<500, or <1,000, IU / mL by Day 2 of treatment.
Owner:EMORY UNIVERSITY
Humanized Anti-Claudin-1 Antibodies and Uses Thereof
ActiveUS20190100586A1Easy to assembleIncrease and decrease stabilityDigestive systemImmunoglobulins against cell receptors/antigens/surface-determinantsUpa scidHepatocellular carcinoma
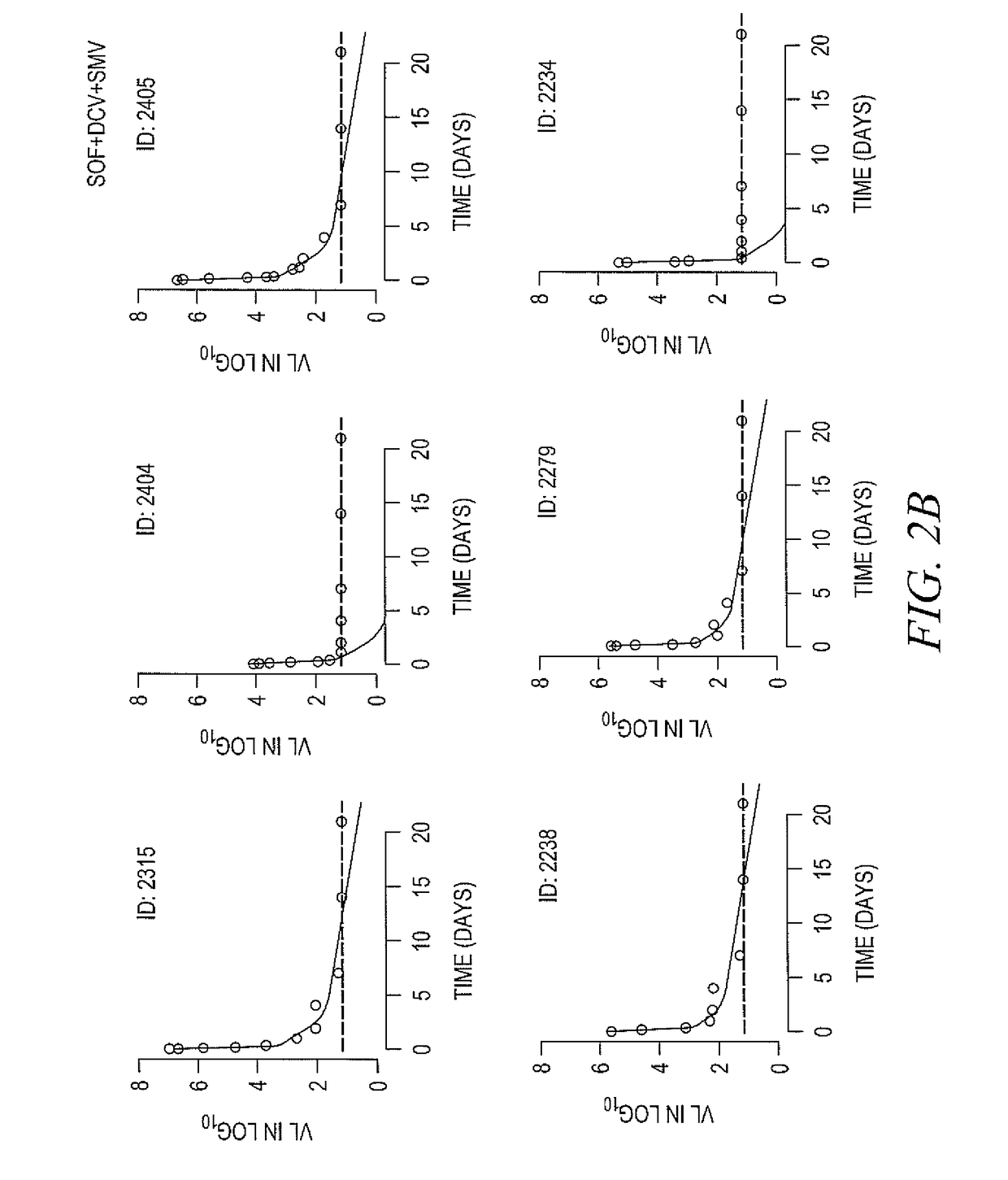
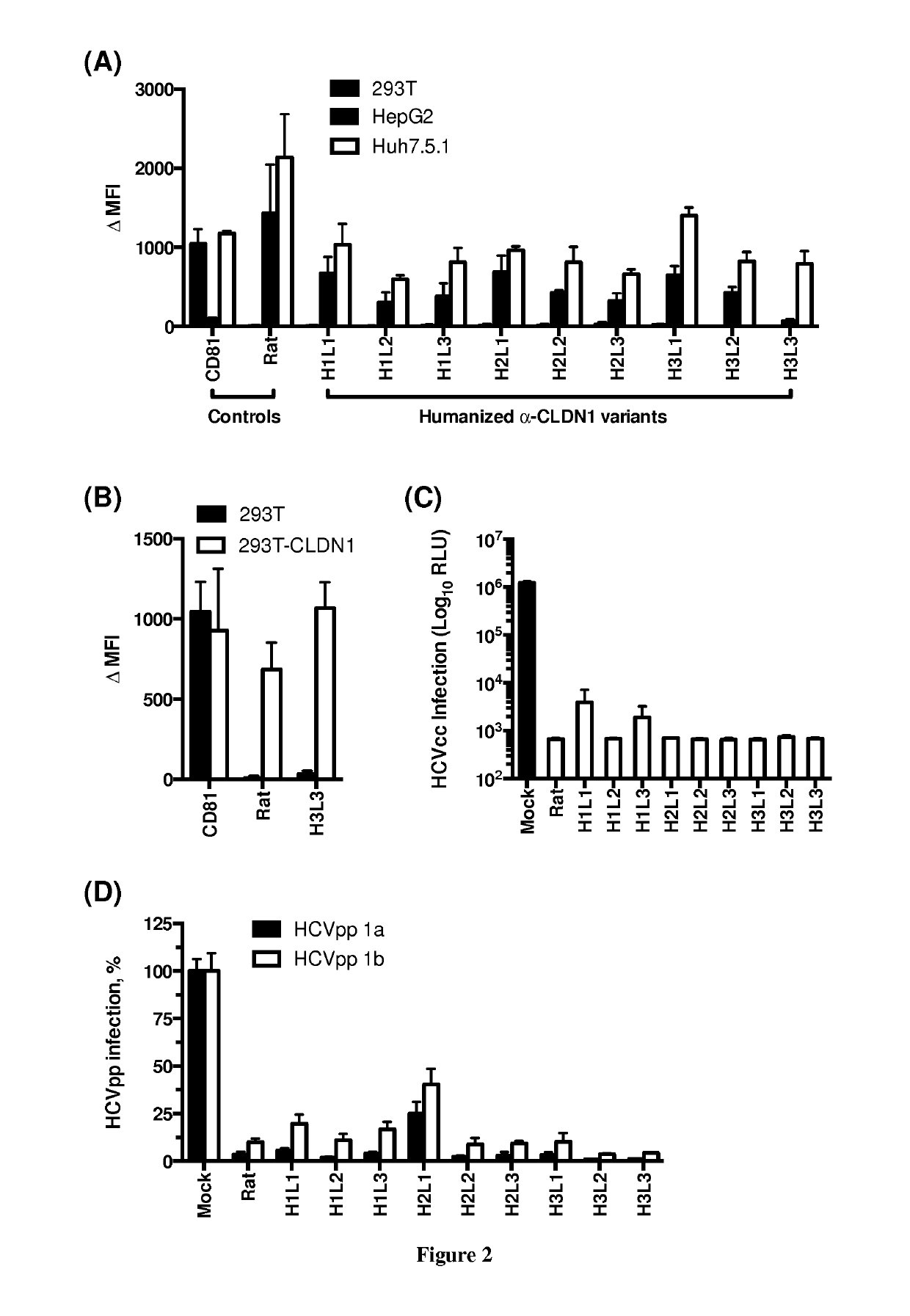
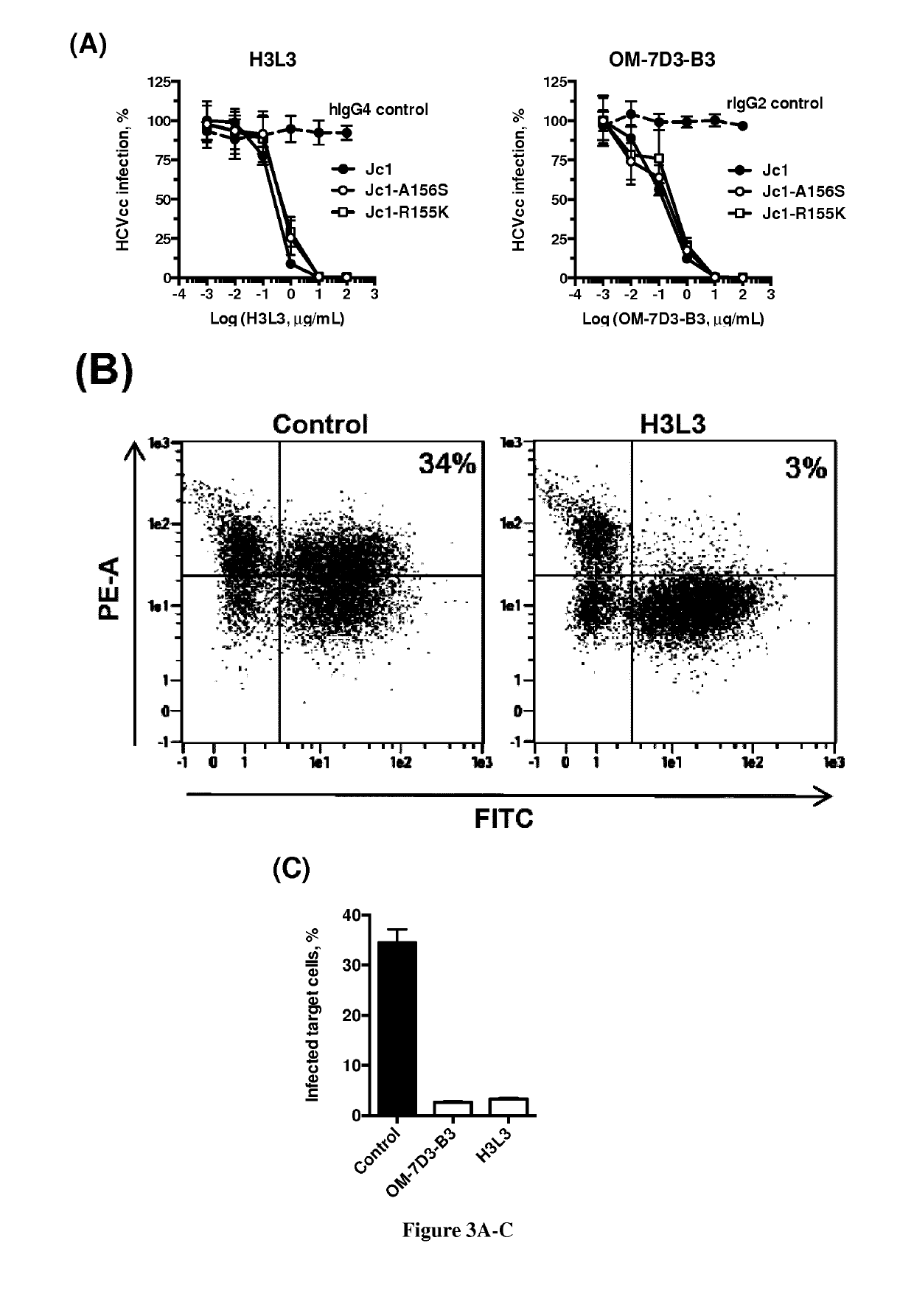
The present invention relates to humanized anti-claudin-1 antibodies and uses thereof. Hepatitis C virus infection is a leading cause of chronic liver disease and a major indication for liver transplantation. The tight junction protein claudin-1 (CLDN1) is an essential entry factor for HCV and a promising target for therapy. For clinical development, the inventors have humanized a rat anti-CLDN1 antibody produced by genetic immunization that prevent HCV infection and also cure chronically infected human liver chimeric mice. The lead humanized anti-CLDN1 antibody (H3L3) pan-genotypically inhibited HCV pseudoparticle infection of primary human hepatocytes (PHH) without detectable escape. H3L3 efficiently inhibited infection by diverse HCV genotype 3 strains and exhibited marked synergy with direct-acting antivirals (DAAs). The inventors also demonstrate that anti-CLDN1 H3L3 cures persistent HCV infection in human-liver chimeric uPA-SCID mice in monotherapy. Thus, the present invention relates to humanized anti-claudin-1 antibodies and uses thereof, in particular for the prevention and treatment of hepatitis C virus infection, virus-induced liver diseases, hepatocellular carcinoma (HCC), nonalcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH).
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Methods for Treating HCV
InactiveUS20180042982A1Avoid side effectsEfficient managementOrganic active ingredientsDipeptide ingredientsShort durationPibrentasvir
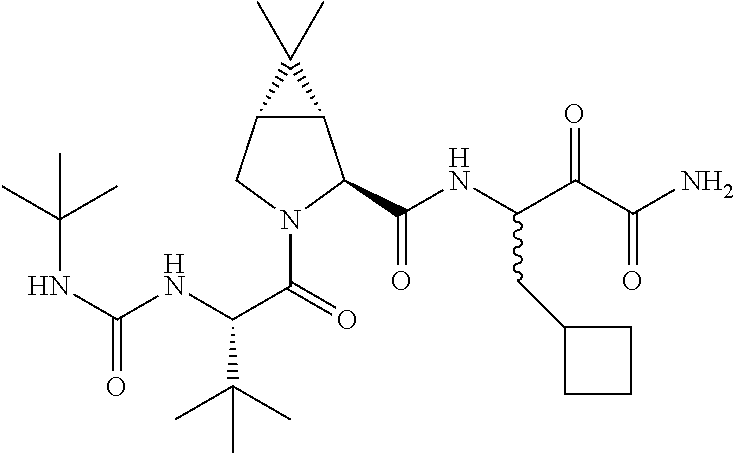
The present invention generally features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 16, 12 or 8 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of interferon, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof. Further, additional compounds such as sofosbuvir, or its pharmaceutically acceptable salt may be used for retreatment of HCV patients who have failed glecaprevir and pibrentasvir combination therapy.
Owner:ABBVIE INC +1
Methods for treating HCV
ActiveUS11484534B2Organic active ingredientsDipeptide ingredientsChemical compoundPharmaceutical Substances
The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of either interferon or ribavirin, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof.
Owner:ABBVIE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com