Method for synthesizing anti-AIDS pharmaceutical efavirenz
An efavirenz and anti-AIDS technology, applied in the field of drug synthesis, can solve problems such as high cost, high risk, and unsatisfactory industrialization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
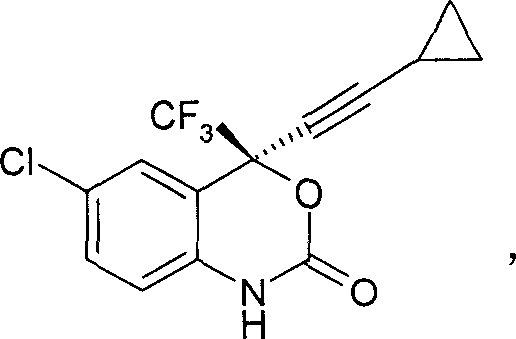
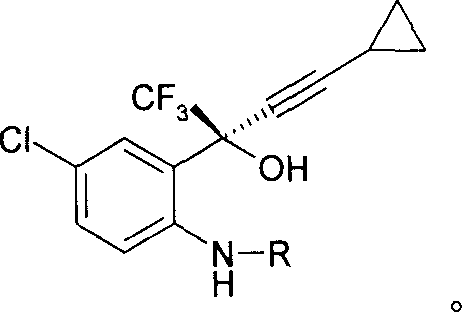
[0022] How to implement the present invention will be further described below in conjunction with specific examples. The following examples are helpful for understanding the present invention, but are not limited to the content of the present invention. Synthesis of (S)-5-chloro-a-(cyclopropylethynyl)-2-[(4-methoxyphenyl)amino]-a-(trifluoromethyl)benzyl alcohol
[0023] method one:
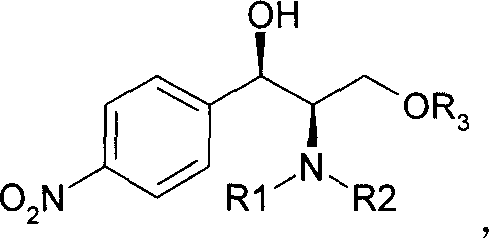
[0024] Ligand (1R, 2R)-1-(p-nitrophenyl)-2-(N, N-dimethylamino)-3-(tert-butyloxy)-1-propanol 180.8 grams ( Add 0.611mol) to 440ml tetrahydrofuran, add a small amount of triphenylmethane (indicator), cool to -10°C, then add butyllithium (1.6M) dropwise to the solution until the solution turns deep red, control the temperature Below -10°C, 40.3 g (0.611 mol) of cyclopropylacetylene was added to the reaction liquid, and the temperature was controlled below -5°C. After the addition, keep the temperature at -5°C and stir for 30 minutes, then cool to -20°C, add 143.5g (0.41ml) of Intermediate I dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com