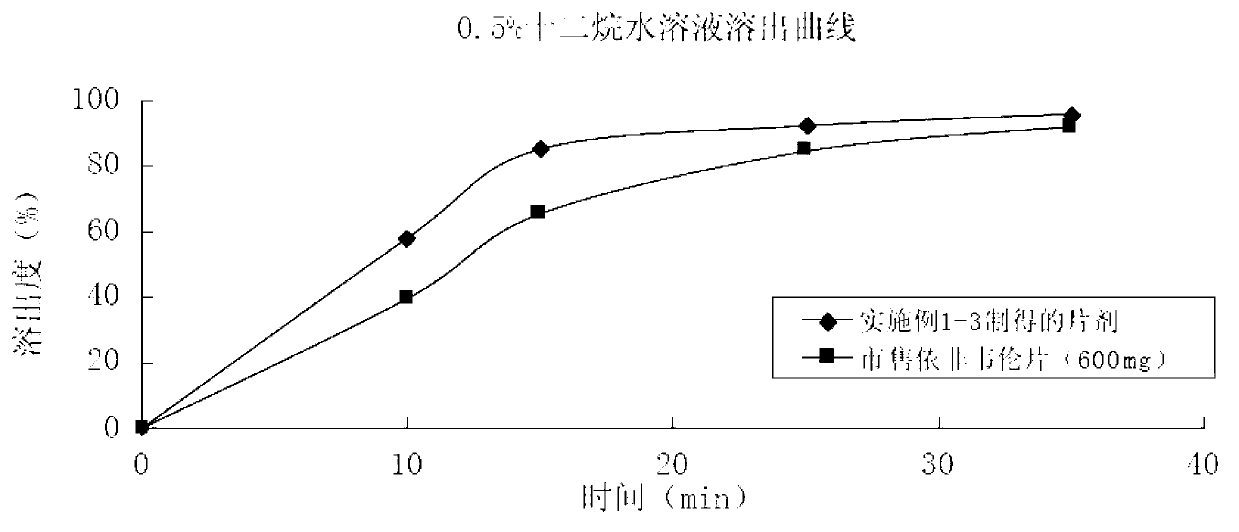
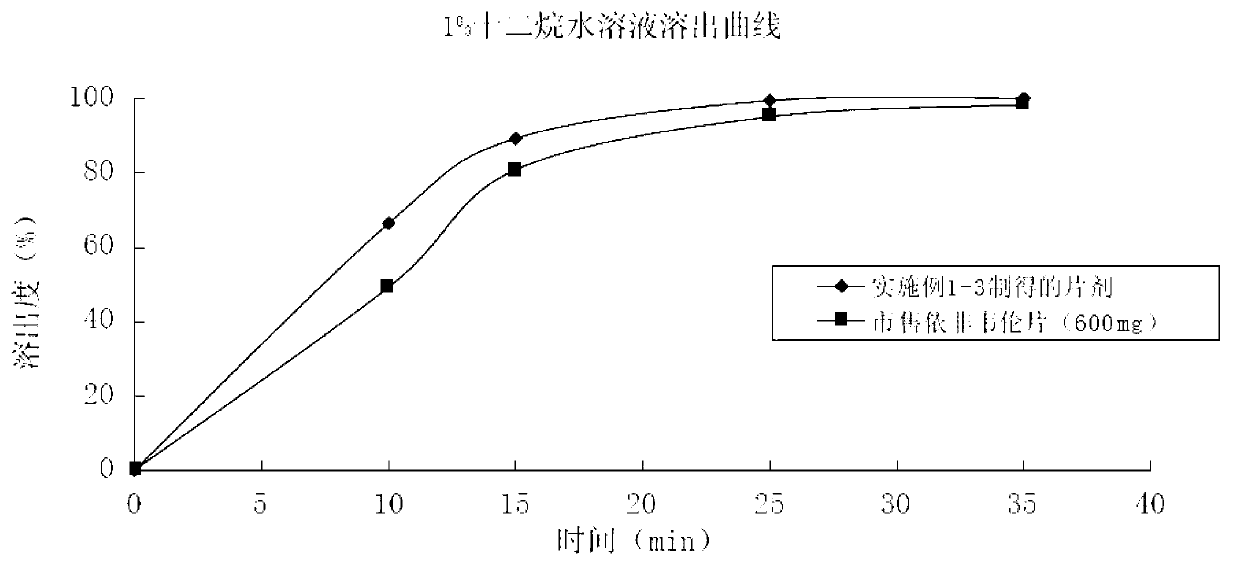
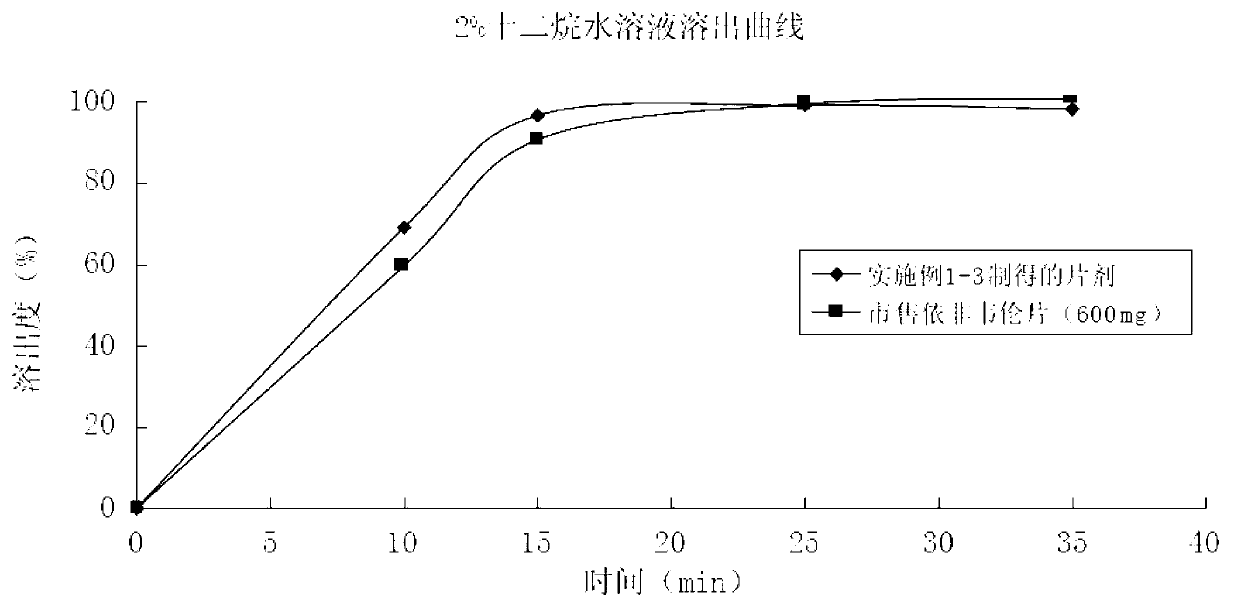
Efavirenz tablet and preparation method thereof
A technology for efavirenz and velen tablets, applied in the field of efavirenz tablets and their preparation, can solve the problems of slow absorption and inability to completely absorb active ingredients and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Weigh the raw materials according to the weights listed in Table 1.
[0026] (2) Dissolve the weighed sodium lauryl sulfate in water to make an aqueous solution of sodium lauryl sulfate; then mix the weighed efavirenz, microcrystalline cellulose, hydroxypropyl cellulose and Add 3 / 10 croscarmellose sodium into the prepared sodium lauryl sulfate aqueous solution and stir to form a soft material. The soft material is granulated in a 18-24 mesh granulation screen. After granulation The granules are sized through a 18-24 mesh screen. Then the magnesium stearate taken by weighing and the remaining croscarmellose sodium and the granulated granules are mixed uniformly and compressed into tablets to obtain 1000 tablets;
[0027] (3) Prepare the weighed hydroxypropyl methylcellulose into a 5% solution with 85% ethanol, and use this solution to coat the tablet prepared in S2 to obtain the finished efavirenz tablet. The average mass of the measured single efavirenz tablet is ...
Embodiment 2
[0031] (1) Weigh the raw materials according to the weights listed in Table 2.
[0032] (2) Dissolve the weighed sodium lauryl sulfate in water to make an aqueous solution of sodium lauryl sulfate; then mix the weighed efavirenz, microcrystalline cellulose, hydroxypropyl cellulose and Add 1 / 2 of the croscarmellose sodium into the prepared sodium lauryl sulfate aqueous solution and stir to form a soft material. The soft material is granulated in a 18-24 mesh granulation screen. After granulation, The granules are sized through a 18-24 mesh screen. Then the magnesium stearate taken by weighing and the remaining croscarmellose sodium and the granulated granules are mixed uniformly and compressed into tablets to obtain 1000 tablets;
[0033] (3) Prepare the weighed hydroxypropyl methylcellulose into a 5% solution with 85% ethanol, and use this solution to coat the tablet prepared in S2 to obtain the finished efavirenz tablet. The measured average mass of a single efavirenz table...
Embodiment 3
[0037] (1) Weigh the raw materials according to the weights listed in Table 3.
[0038] Table 3 Formula Composition of Efavirenz Tablets (C)
[0039]
[0040] (2) Dissolve the weighed sodium lauryl sulfate in water to make an aqueous solution of sodium lauryl sulfate; then mix the weighed efavirenz, microcrystalline cellulose, hydroxypropyl cellulose and Add 1 / 2 of the croscarmellose sodium into the prepared sodium lauryl sulfate aqueous solution and stir to form a soft material. The soft material is granulated in a 18-24 mesh granulation screen. After granulation, The granules are sized through a 18-24 mesh screen. Then, the weighed magnesium stearate and the remaining croscarmellose sodium were uniformly mixed with the granulated granules and compressed into 1000 tablets.
[0041] (3) Prepare the weighed hydroxypropyl methylcellulose into a 5% solution with 85% ethanol, and use this solution to coat the tablet prepared in S2 to obtain the finished efavirenz tablet. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com