Tenofovir, lamivudine and efavirenz triple compound pellets and preparation method thereof
A technology of lamivudine pellets and tenofovir, which is applied in pill delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as delamination, poor compressibility, and poor plasticity of pellets. Achieve good mixing uniformity, reduce the amount of auxiliary materials, and good formability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
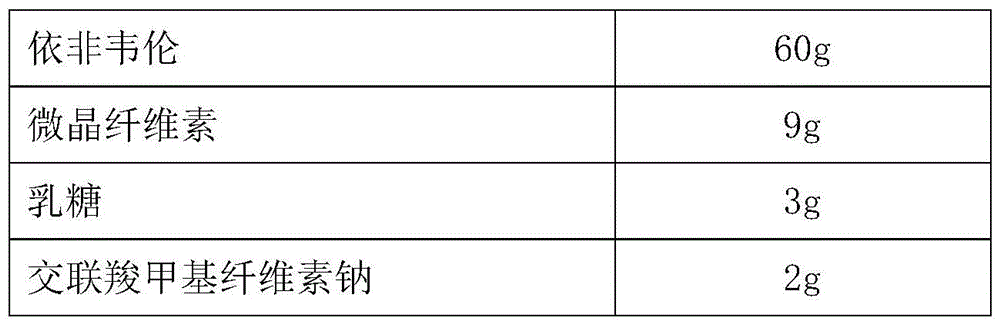
[0046] (a) preparation of tenofovir DF coated pellets
[0047] Tenofovir DF
30g
6g
2g
2.5g
2% aqueous solution of povidone K30
Q.S.
Commercially available hydroxypropyl methylcellulose coating solution
Q.S.
100 pieces
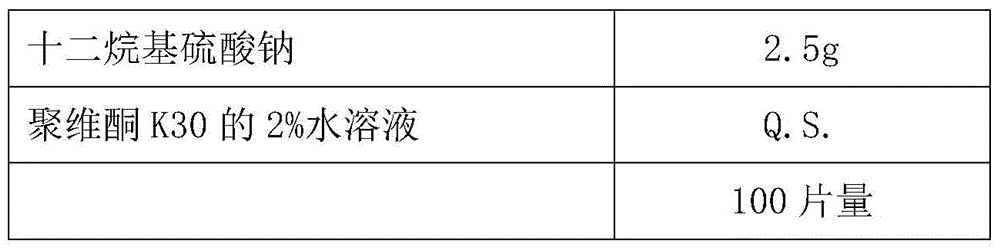
[0048] Preparation method: Weigh the prescription amount of raw materials and auxiliary materials, mix them evenly, use 2% aqueous solution of povidone K30 as the adhesive to make soft materials, extrude and spheronize to prepare tenofovir DF drug-containing pellet cores, and sieve 30-40 mesh ready to use. Take the above-mentioned drug-containing pellet cores in a fluidized bed, prepare a commercially available finished hydroxypropyl methylcellulose coating solution, and coat an isolation coat. The weight gain of the coating is about 6%, and tenofovir DF coated pellets are prepared.
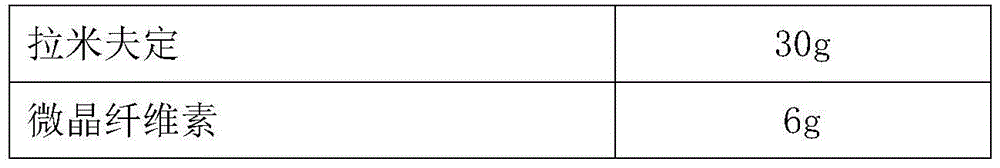
[0049] (b) preparation of lamivudine co...
Embodiment 2
[0058] (a) preparation of tenofovir DF coated pellets
[0059] Tenofovir DF
30g
8g
2.5g
2% aqueous solution of povidone K30
Q.S.
Commercially available hydroxypropyl methylcellulose coating solution
Q.S.
100 pieces
[0060] Preparation method: Weigh the prescription amount of raw materials and auxiliary materials, mix them evenly, use 2% aqueous solution of povidone K30 as the adhesive to make soft materials, extrude and spheronize to prepare tenofovir DF drug-containing pellet cores, and sieve 30-40 mesh ready to use. Take the above-mentioned drug-containing pellet cores in a fluidized bed, prepare a commercially available finished hydroxypropyl methylcellulose coating solution, and coat an isolation coat. The weight gain of the coating is about 6%, and tenofovir DF coated pellets are prepared.
[0061] (b) preparation of lamivudine coated pellets
[0062...
Embodiment 3
[0069] (a) preparation of tenofovir DF coated pellets
[0070] Tenofovir DF
30g
6g
2g
2.5g
2% aqueous solution of povidone K30
Q.S.
Commercially available hydroxypropyl methylcellulose coating solution
Q.S.
100 pieces
[0071] Preparation method: Weigh the prescription amount of raw materials and auxiliary materials, mix them evenly, use 2% aqueous solution of povidone K30 as the adhesive to make soft materials, extrude and spheronize to prepare tenofovir DF drug-containing pellet cores, and sieve 30-40 mesh ready to use. Take the above-mentioned drug-containing pellet cores in a centrifugal granulation coating machine, prepare a commercially available finished hydroxypropyl methylcellulose coating liquid and coat an isolation coat, and the weight of the coating will increase by about 6%, to prepare tenofovir DF coated pellets .
[0072] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com