Preparation method of intermediate cyclopropyl acetylene of anti-AIDS (acquired immune deficiency syndrome) drug efavirenz
A cyclopropylacetylene, anti-AIDS technology, applied in the direction of dehydrohalogenation preparation, hydrocarbon production from halogen-containing organic compounds, phosphorus halide/oxyhalide, etc., can solve the problem of cooling liquid temperature not being too low, crystallization, decompression precision Distillation vacuum limit and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
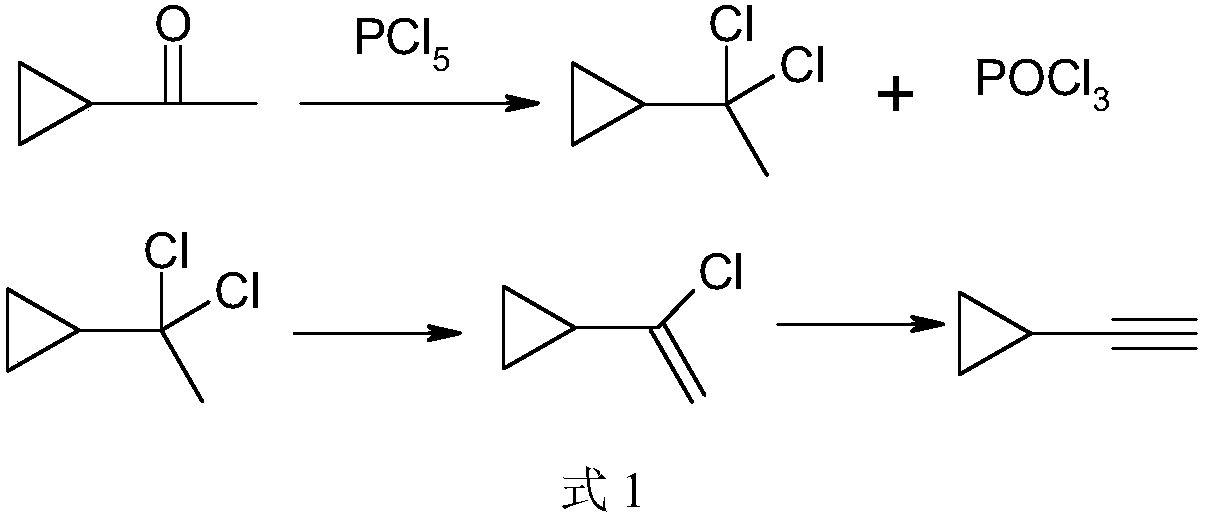
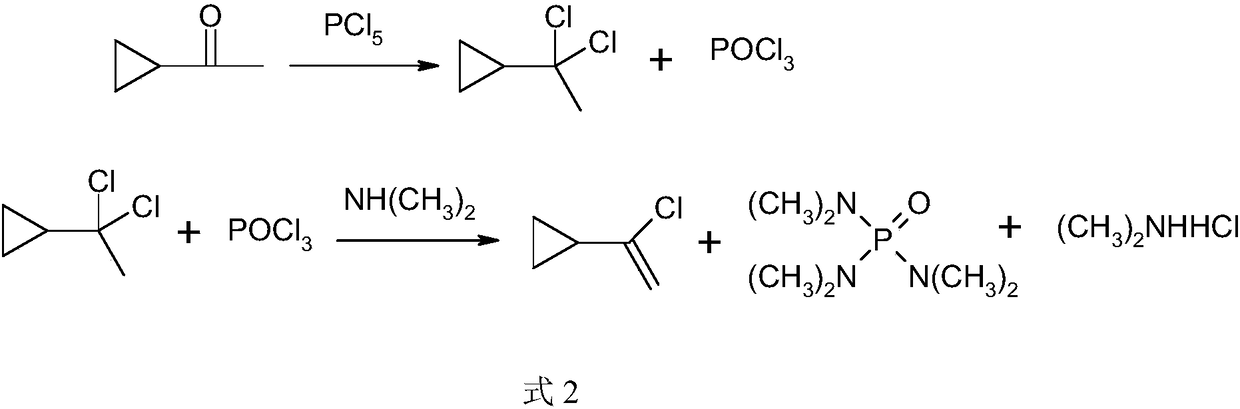
[0026] Add ethylcyclohexane (500ml) and phosphorus pentachloride (204.1g, 0.98mol) into a 1000ml reaction flask, add 2g of triethylamine, start stirring, adjust the temperature to 40-50°C, then add cyclopropane dropwise methyl ketone (84.1 g, 1 mol), the dropwise addition was completed, and the temperature was kept for 1 hour. The reaction solution obtained above is rectified under reduced pressure, the pressure is controlled at 3.8-4.0kPa, the reflux ratio is 2, the temperature of the kettle is controlled to be ≤80°C, the fraction with the temperature of the tower top ≤30°C is collected, and the by-product phosphorus oxychloride produced by the reaction is recovered ; The residual liquid in the tower kettle was transferred to a 1000ml reaction flask, and 120 grams of triethylamine was added to the above reaction flask, the temperature was raised to reflux temperature, and the temperature was kept for 1 hour. Atmospheric distillation obtained 60.1 grams of α-chlorovinylcyclopr...
Embodiment 2
[0028]Add chlorobenzene (500ml) and phosphorus pentachloride (218.7g, 1.05mol) into the reaction flask, add 2g of triethylamine, start stirring, adjust the temperature to 30-40°C, then add cyclopropylmethyl ketone dropwise (84.1 g, 1 mol), the dropwise addition was completed, and the temperature was kept for 2 hours. The obtained reaction solution is rectified under reduced pressure, the pressure is controlled at 2.5-2.7kPa, the reflux ratio is 3, the temperature of the kettle is controlled to be ≤ 70°C, the fraction with the temperature at the top of the tower ≤ 25°C is collected, and the by-product phosphorus oxychloride produced by the reaction is recovered; The residual liquid in the tower kettle was transferred to a 1000ml reaction flask. Add 120 grams of triethylamine in above-mentioned reaction bottle, be warming up to reflux temperature, keep warm for 10 hours. Atmospheric distillation to obtain 62.5 grams of α-chlorovinyl cyclopropane; using reactive distillation tec...
Embodiment 3
[0030] Add ethylbenzene (500ml) and phosphorus pentachloride (220.5 grams, 1.06mol) into the reaction flask, add 2 grams of pyridine, start stirring, adjust the temperature to 20-30°C, then add cyclopropyl methyl ketone (84.1 gram, 1mol), the dropwise addition was completed and incubated for 3 hours. The obtained reaction solution is rectified under reduced pressure, the pressure is controlled at 2.5-2.7kPa, the reflux ratio is 3, the temperature of the kettle is controlled to be ≤70°C, the fraction with the temperature of the tower top ≤25°C is collected, and the by-product phosphorus oxychloride produced by the reaction is recovered; The residual liquid in the tower kettle was transferred to a 1000ml reaction flask. Add 120 grams of triethylamine in above-mentioned reaction bottle, be warming up to reflux temperature, keep warm for 10 hours. Distill at atmospheric pressure to obtain 64.8 grams of α-chlorovinyl cyclopropane; adopt reactive distillation technology, add 125 gr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com