Efavirenz preparation adopting micronization technology
A technology for efavirenz and tablets, which is applied in the field of tableting or capsules to achieve the effects of improving solubility and reducing usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
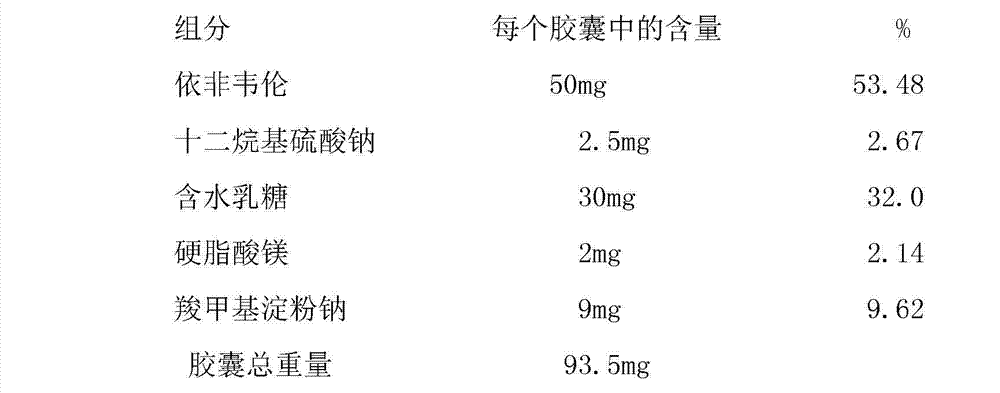
[0019] Embodiment 1: wet granulation 50mg capsule preparation
[0020] Preparation method: Mix efavirenz with sodium carboxymethyl starch whose particle size at 90% cumulative volume is controlled below 66um, then add sodium lauryl sulfate aqueous solution and carry out wet granulation. The wet material is then dried in a fluid bed, drying tray or other suitable desiccant. The dried granules can then be milled to a suitable particle size distribution prior to mixing with the other ingredients. The mixture is then filled into two hard gelatin capsule shells.
[0021]
[0022]
Embodiment 2
[0023] Embodiment 2: wet granulation 50mg capsule preparation
[0024] Preparation method: Mix efavirenz with a particle size above 66um at 90% cumulative volume and sodium carboxymethyl starch, then add sodium lauryl sulfate aqueous solution and carry out wet granulation. The wet material is then dried in a fluid bed, drying tray or other suitable desiccant. The dried granules can then be milled to a suitable particle size distribution prior to mixing with the other ingredients. The mixture is then filled into two hard gelatin capsule shells.
[0025]
Embodiment 3
[0026] Example 3: Wet Granulation 50mg Tablet Formulation
[0027] Preparation method: use sodium lauryl sulfate aqueous solution to granulate efavirenz, lactose, sodium carboxymethyl starch and microcrystalline cellulose whose particle size at 90% cumulative volume is controlled below 66um. The wet material is then dried in a fluid bed, drying pan or other suitable drying place. The dried granules can then be milled to achieve the desired particle size distribution, the magnesium stearate added and the mixture compressed to form tablets. Tablets may be coated, if desired.
[0028]
[0029]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com