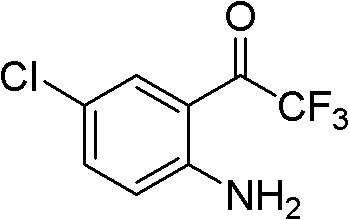
Simple, convenient and high-efficiency synthesis method of 4-chloro-2-trifluoroacetylaniline and analogs thereof
A technique for the synthesis of trifluoroacetylaniline and its synthesis method, which is applied in the field of key intermediates for the synthesis of the HIV reverse transcriptase inhibitor efavirenz, and can solve the problems of unfavorable large-scale industrial production, high requirements for equipment, and low synthesis efficiency. Achieve the effects of simple operation, low yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0063] Example 1 Preparation of 3-hydroxy-3-trifluoromethylindol-2-one:
[0064] Aniline (1.4g, 15mmol) was dissolved in benzene (20mL), and methyl trifluoropyruvate (23.4g, 150mmol) was added dropwise. After the dropwise addition, the temperature was raised to about 130°C in an oil bath. After the reaction was carried out for 18 hours, the oil bath was removed, and it was naturally cooled to room temperature. A large amount of white solids were seen to precipitate out. The white solids were filtered off, then washed with petroleum ether, and dried to obtain the product (2.6 g, yield 78%), which did not require further purification The next reaction can be carried out.
[0065] 1 HNMR (Acetone-d 6 , 400MHz) δ6.51(s, 1H), 7.03(d, J=5.7Hz, 1H), 7.13(t, J=11.4Hz, 5.7Hz, 1H), 7.42(t, J=11.4Hz, 5.7Hz , 1H), 7.52(d, J=5.7Hz, 1H), 9.76(s, 1H).
[0066] HRMS. Calculated value C 9 h 6 f 3 NO 2 : 217.0351. Measured value: 217.0353.
Embodiment 25
[0067] Example 2 Preparation of 5-chloro-3-hydroxy-3-trifluoromethylindol-2-one:
[0068] 3-Hydroxy-3-trifluoromethylindol-2-one (3.3g, 15.2mmol) and NCS (2.5g, 18.7mmol) were dissolved in DMF (50mL), and zirconium tetrachloride (180mg, 5% eq) As a catalyst, the temperature of the oil bath was raised to 50° C., and the reaction was completed after 48 hours. After the reaction was complete, ethyl acetate was added for extraction, washed with saturated sodium bicarbonate, washed with water and saturated brine in sequence, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the crude product could be recrystallized from toluene / ethanol (20:1) to obtain the product (3.7 g, yield 98%).
[0069] 1 H NMR (500MHz, acetone-d 6 )δ6.77(s, 1H), 7.08(d, 1H, J=8.0Hz), 7.47(dd, 1H, J=1.5Hz, J=8.5Hz), 9.96(s, 1H);
[0070] 19 F NMR (acetone-d6) δ79.99;
[0071] MS (GC) m / z 251 (M + ).
Embodiment 34
[0072] Example 3 Preparation of 4-chloro-2-trifluoroacetylaniline:
[0073] At room temperature, 5-chloro-3-hydroxy-3-trifluoromethylindol-2-one (251mg, 1mmol) was dissolved in acetonitrile (10mL), water (3mL) was added, and KOH (280mg, 5mmol ), after fully dissolved, add K at one time 3 [Fe(CN) 6 ] (990mg, 3mmol), after the reaction of the raw materials was complete, ethyl acetate was added for extraction, the organic phase was washed with water and saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain the product (211.9mg, yield 95%).
[0074] 1 H NMR (300MHz, CDCl 3 )δ6.46 (brs, 2H), 6.70 (d, 1H, J=9.0Hz), 7.32 (dd, 1H, J=2.1Hz, 9.0Hz), 7.71 (d, 1H, J=2.1Hz);
[0075] MS (ESI) m / z 222 (M-H);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com