Efavirenz, lamivudine and tenofovir disoproxil fumarate triple compound tablet middle tablet and preparation method thereof
A technology of tenofovir disoproxil fumarate and efavirenz, which is applied in the field of pharmaceutical preparations, can solve the problems of broken pellets, uncontrollable degree and proportion of broken pellets, and difficult to achieve degradation, so as to prevent degradation, Good dissolution, moderately sized effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
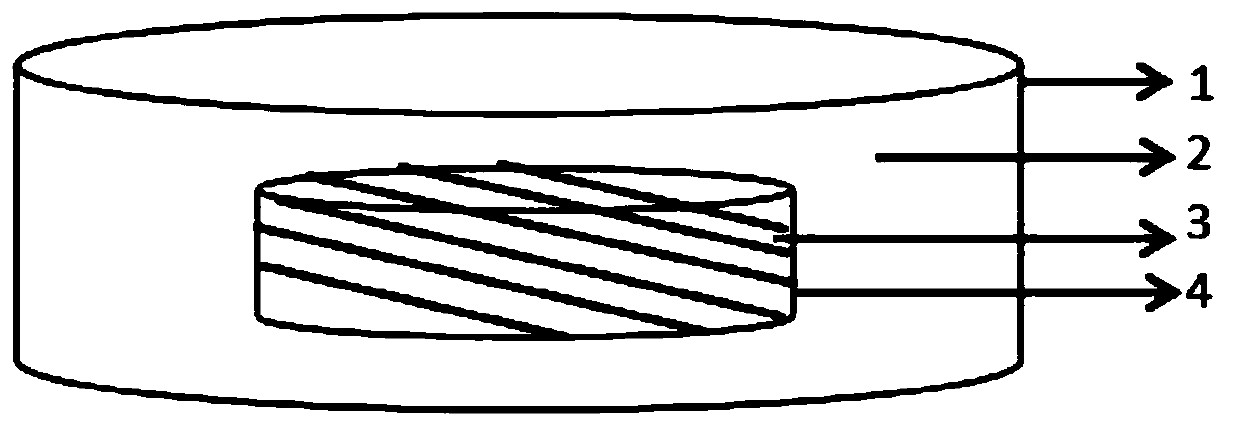
[0041] Preparation of core part:
[0042] The tenofovir disoproxil fumarate bulk drug is processed through a 40-60 mesh sieve. Tenofovir disoproxil fumarate 150g (50%), anhydrous calcium hydrogen phosphate 127.5g (42.5%), the pregelatinized starch 3g (1%) of adding amount in clean container; Put it into the wet mixing granulator, start stirring and mixing for 3-10 minutes; add an appropriate amount of purified water into the wet mixing granulator, start stirring and shearing granulation, the granulation time is 1.5-3 minutes, until the Soft materials that are suitable for hardness and softness; 18-24 mesh nylon screens are installed on the oscillating granulator, and the soft material prepared in the previous step is slowly added to the oscillating granulator, and wet granules are prepared and put into a drying oven for drying at a temperature of 60°C. Install 18-24 mesh sieves on the swinging granulator, pass the dried granules through the 18-24 mesh swinging granulator for ...
Embodiment 2
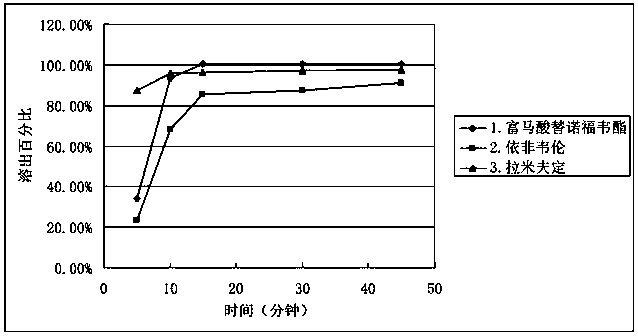
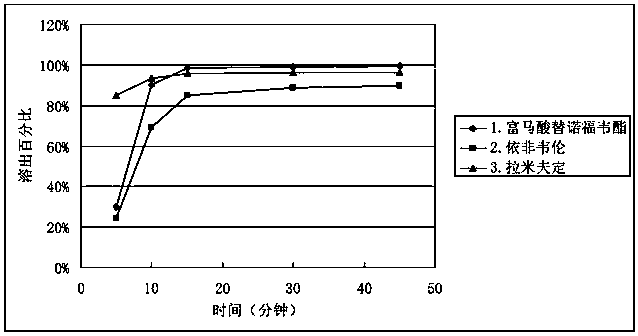
[0046] Mix the raw and auxiliary materials according to the ratio of the prescription amount, compress the common tablet and then coat it, and compare it with the sample content of the tablet-in-tablet according to the present invention at 0 o'clock and long-term 3 months respectively under the same conditions. The results showed that there was no significant difference in the content of ordinary tablets and tablets-in-tablets at 0 o'clock, but the content of tenofovir disoproxil fumarate in ordinary tablets decreased significantly in the long-term for 3 months, while the content of tenofovir disoproxil fumarate in tablets-in-tablets The content of fovir dipivoxil remained almost unchanged, the results are shown in Table 1.
[0047] Table 1 Content comparison of tenofovir disoproxil fumarate at 0 hours and long-term for 3 months
[0048] 0 o'clock long term 3 months Triple Compound Ordinary Tablets 98.9% 90.1% Triple compound tablet-in-tablet 100.3% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com