Clinical data management system
A management system and clinical data technology, applied in the direction of data processing applications, patient-specific data, instruments, etc., can solve problems such as difficulties in the center, lack of substantial management, and high costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] specific implementation
[0024] The technical scheme that the present invention takes for solving the technical problem existing in known technology is:
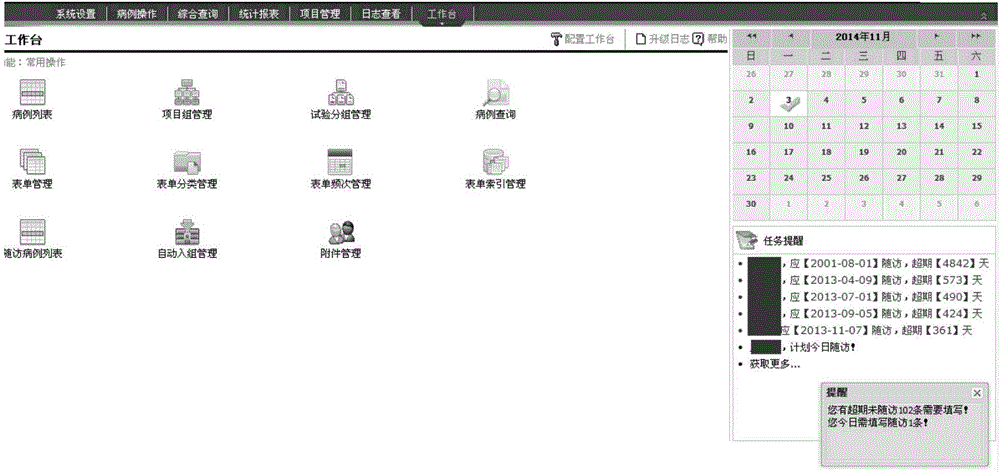
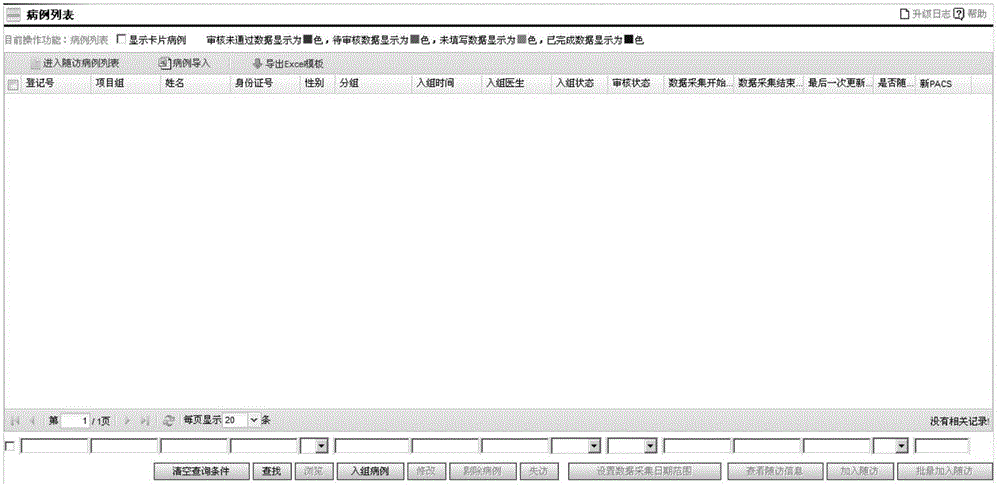
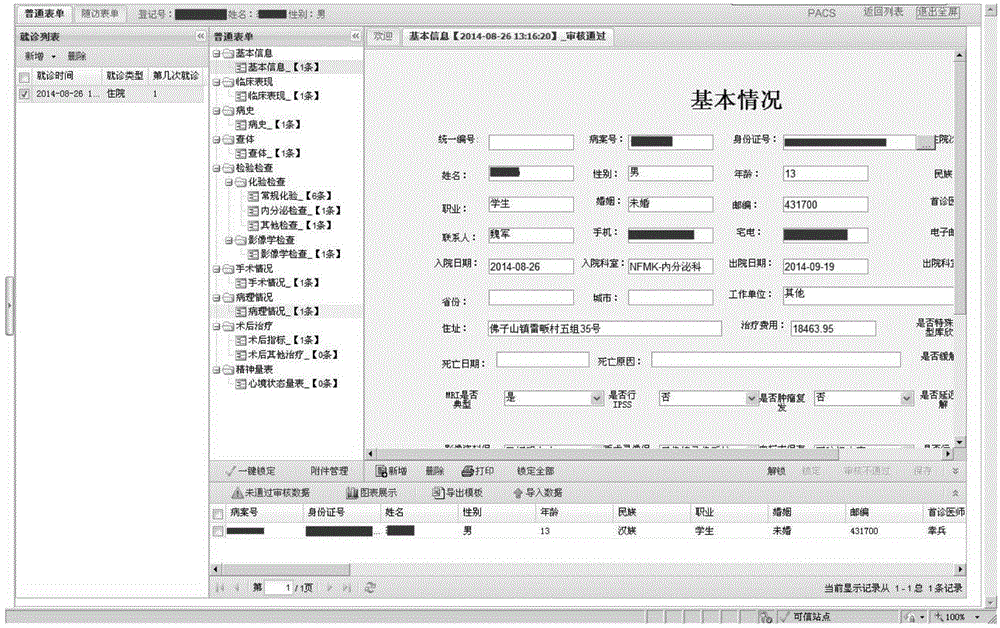
[0025] A clinical data management system is provided with a workbench, a medical record management module, a follow-up management module, a project management module, a comprehensive query module, and an automatic collection task management module. The following specifically describes each module.
[0026] The workbench is the working interface of the user's routinely used medical record management module, follow-up management module, project management module, comprehensive query module, and automatic collection task management module. It provides users with common operation shortcuts and calendar functions. Users can configure The workbench can add or delete the shortcuts of the workbench by itself, and a maximum of 16 shortcut icons can be displayed; in addition, the follow-up reminder information of the case wil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com