Calcitriol injection and preparation method thereof
A technology for calcitriol and injections, which is applied in bone diseases, pharmaceutical formulations, medical preparations with non-active ingredients, etc., and can solve the problems of monotonous dosage forms, calcitriol sensitivity to light and air, soft capsules and gelatin The stability of pills is not good and other problems, and the effect of improving bioavailability is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 calcitriol injection
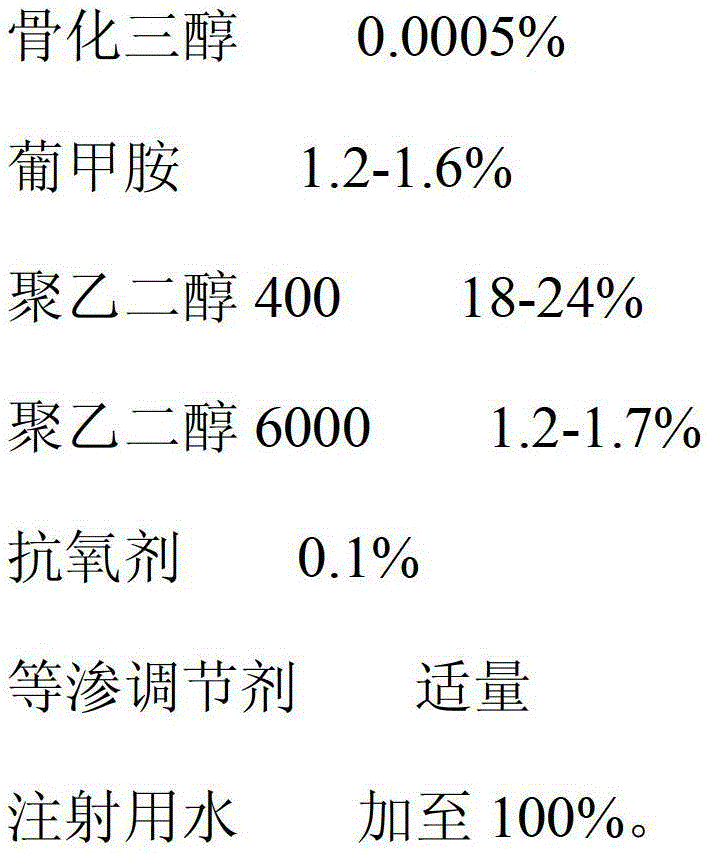
[0026] The prescription is:
[0027]
[0028] The preparation method is:
[0029] 1) Weigh 70% of the prescribed amount of distilled water, add the prescribed amount of polyethylene glycol 400, and stir evenly; continue to add the prescribed amount of meglumine and polyethylene glycol 6000, and stir at 45°C for 15 minutes until completely dissolved;
[0030] 2) Raise the temperature to 60°C, add the prescribed amount of calcitriol at a stirring speed of 500rpm, and continue stirring for 20 minutes until it is completely dissolved;
[0031] 3) Add the prescribed amount of sodium sulfite and appropriate amount of sodium chloride, stir to dissolve; then add 0.1% activated carbon of the total mass of the injection, stir evenly, let it stand, and filter to remove the carbon powder;
[0032] 4) After measuring and adjusting the pH value of the solution, add water for injection to the full amount; filter through 0.45 μm and 0.22 μm micr...
Embodiment 2
[0033] Embodiment 2 calcitriol injection
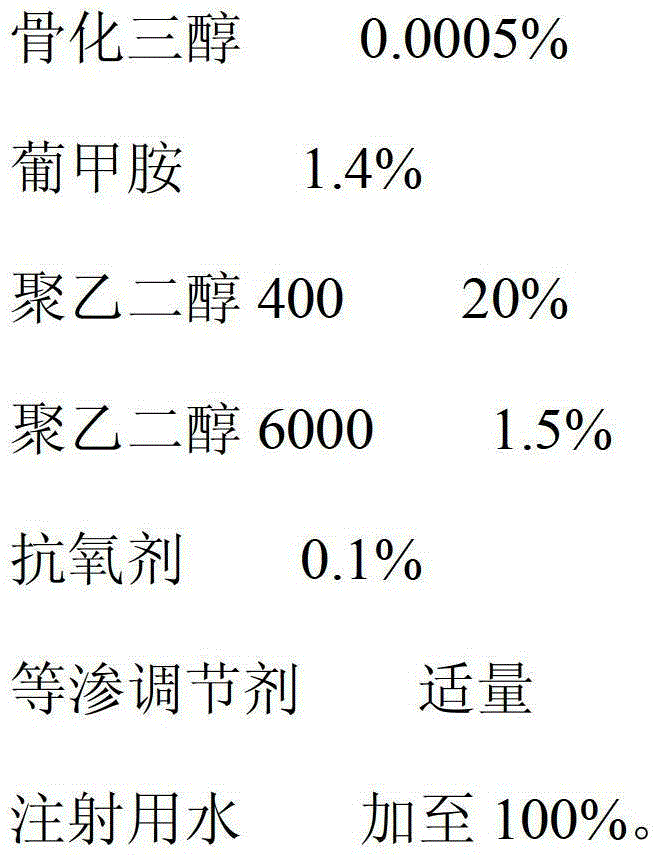
[0034] The prescription is:
[0035]
[0036] The preparation method is:
[0037] 1) Weigh 80% of the prescribed amount of distilled water, add the prescribed amount of polyethylene glycol 400, and stir evenly; continue to add the prescribed amount of meglumine and polyethylene glycol 6000, and stir at 55°C for 15-25 minutes until completely dissolved;
[0038] 2) Raise the temperature to 70°C, add the prescribed amount of calcitriol at a stirring speed of 1000rpm, and continue stirring for 40 minutes until it is completely dissolved;
[0039] 3) Add the prescribed amount of sodium bisulfite and an appropriate amount of glucose, stir to dissolve; then add 0.1% activated carbon of the total mass of the injection, stir evenly, let stand, and filter to remove the carbon powder;
[0040] 4) After measuring and adjusting the pH value of the solution, add water for injection to the full amount; filter through 0.45 μm and 0.22 μm microp...
Embodiment 3
[0041] Embodiment 3 calcitriol injection
[0042] The prescription is:
[0043]
[0044]
[0045] The preparation method is:
[0046] 1) Weigh 75% of the prescribed amount of distilled water, add the prescribed amount of polyethylene glycol 400, and stir evenly; continue to add the prescribed amount of meglumine and polyethylene glycol 6000, and stir at 50°C for 20 minutes until completely dissolved;
[0047] 2) Raise the temperature to 65°C, add the prescribed amount of calcitriol at a stirring speed of 800rpm, and continue stirring for 30 minutes until it is completely dissolved;
[0048] 3) Add the prescribed amount of sodium metabisulfite and appropriate amount of mannitol, stir to dissolve; then add 0.1% activated carbon of the total mass of the injection, stir evenly, let stand, and filter to remove the carbon powder;
[0049] 4) After measuring and adjusting the pH value of the solution, add water for injection to the full amount; filter through 0.45 μm and 0.22...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com