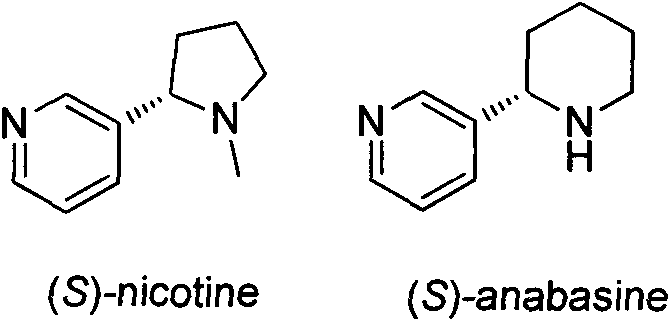
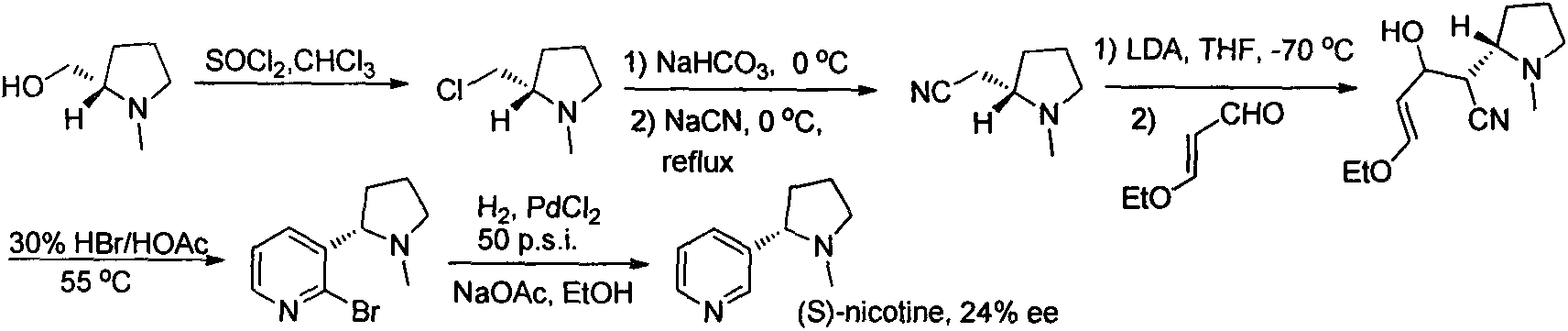
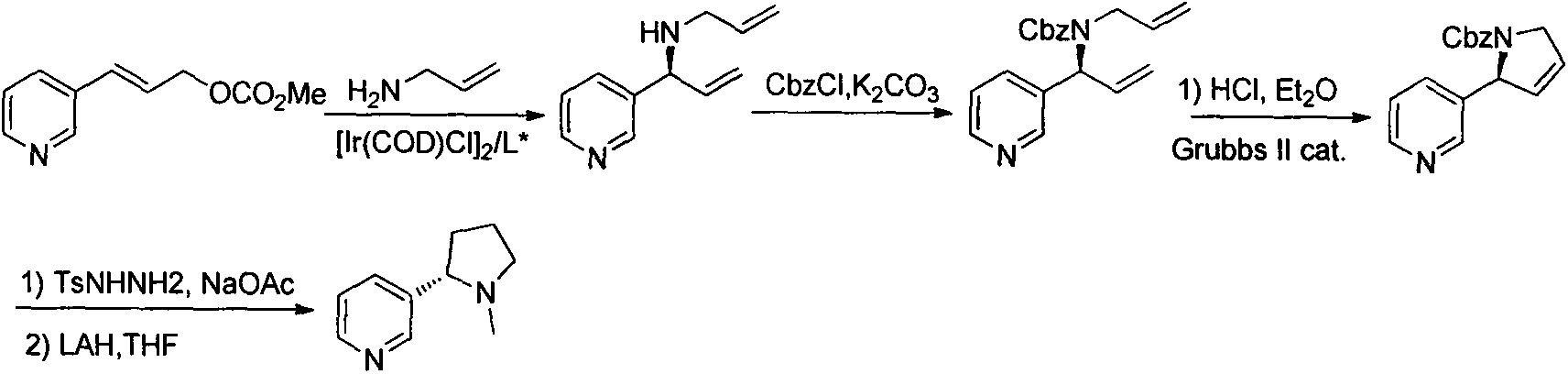
Asymmetric synthesis method for botanical pesticide nicotine and anabasine
A technology of botanical pesticides and anabasine, applied in the direction of organic chemistry, can solve the problems of heavy post-processing workload and high cost of chiral separation methods, and achieve the effects of low cost, stable operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The present invention will be described in further detail below through examples, which do not limit the protection scope of the present invention.
[0020] For the experimental methods that do not indicate specific conditions in the examples, usually follow the conventional conditions and the conditions described in the manual, or according to the conditions suggested by the manufacturer; the materials, reagents, etc. used, if no special instructions, can be purchased from commercial sources. get.
[0021] 1) Synthesis of intermediates 1 and 2
[0022] In a three-neck round bottom flask, 2,5-dibromopyridine (2.37 g, 10 mmol) was added under argon protection and dissolved in 25 ml of ether. The solution was stirred and cooled to -78°C, then slowly added dropwise n-BuLi n-hexane solution (1.6M, 7.5ml), kept at -78°C during the dropwise addition, continued to stir at -78°C for 30min after the dropwise addition, and then slowly dropped Add 1-Boc-2-pyrrolidone (1.85g, 10m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com