Preparation method of Z-1,1,1,4,4,4-hexafluoro-2-butene
A Z-2, Z-1 technology, applied in Z-1, can solve the problems of pollution, corrosion, serious pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
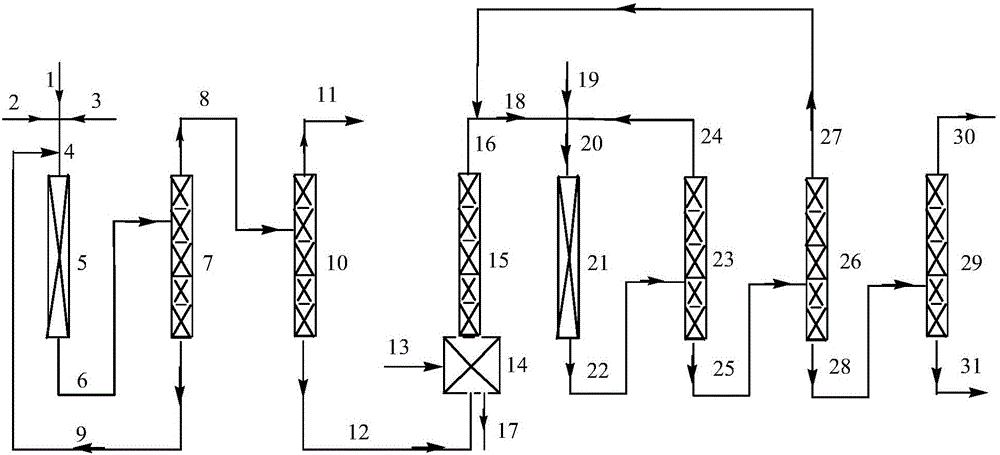
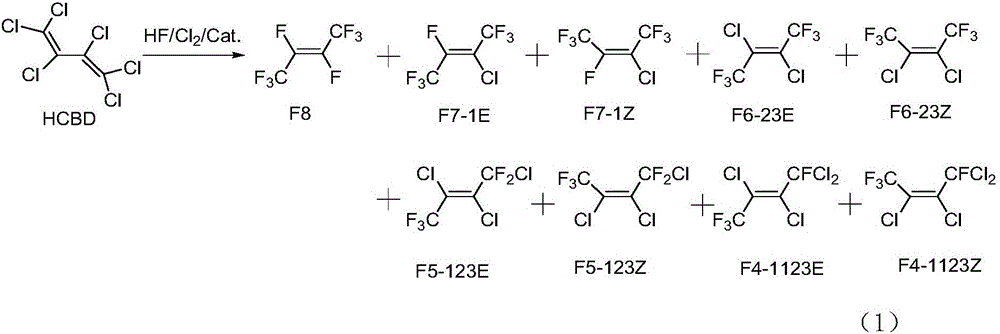
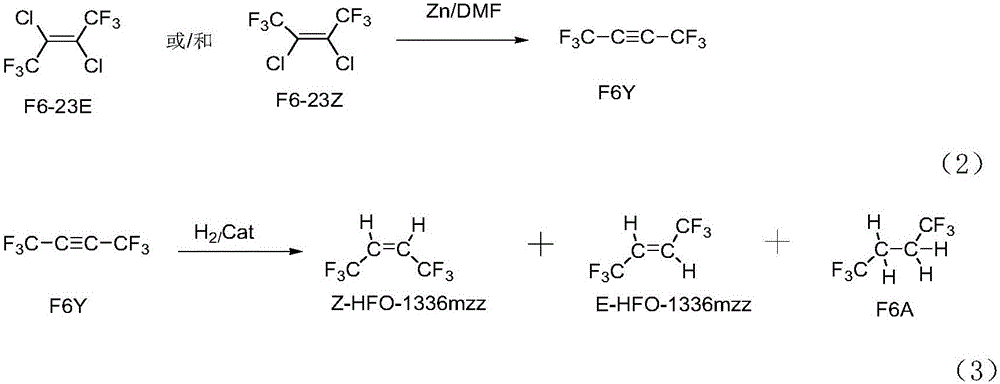
Image
Examples
Embodiment 1
[0063] Preparation of fluorination catalyst: Dissolve chromium nitrate in water, add precipitant ammonia water at 60°C, control the pH of the solution between 7.5 and 8.5, make it fully precipitate under stirring conditions, filter the formed slurry, and use Wash with ion water until neutral, then dry at 150°C for 12 hours to obtain chromium hydroxide. According to the mass percentages of tetravalent chromium ions and iron elements being 95% and 5%, the above-mentioned chromium hydroxide and iron hydroxide are uniformly mixed, pressed and molded to obtain a catalyst precursor, and then the catalyst precursor is placed in a nitrogen atmosphere. After calcination at 450°C for 10 hours, activate at 300°C with a mixed gas of hydrogen fluoride and hydrogen at a molar ratio of 10:1 for 10 hours, and at 300°C with a mixed gas atmosphere of chlorine and nitrogen with a molar ratio of 1:10 Oxidation for 12 hours to obtain a fluorination catalyst.
[0064] A tubular reactor made of Inc...
Embodiment 2
[0066] The same operation as in Example 1, the difference is that "according to the mass percentage of tetravalent chromium ion and iron element is 95% and 5%, the chromium hydroxide and iron hydroxide are mixed homogeneously" to "according to the tetravalent The mass percent composition of chromium ion and zinc element is 95% and 5%, chromium hydroxide and zinc hydroxide are mixed " homogeneously ", and the chlorine that oxidizes the catalyst is changed into the oxygen of the amount of equal substance, the result is shown in the table 1.
Embodiment 3
[0068] The same operation as in Example 1, the difference is that the ferric hydroxide in the fluorination catalyst precursor is changed to zinc hydroxide of equal quality and the chlorine gas that is oxidized to the catalyst is changed to the ozone of the amount of the same substance, the results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com