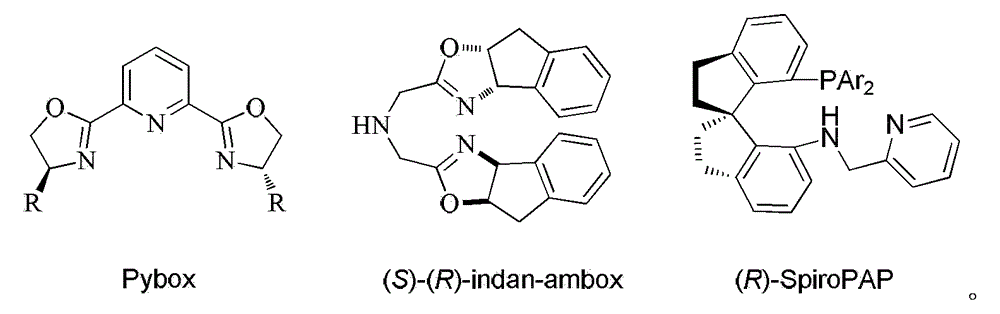
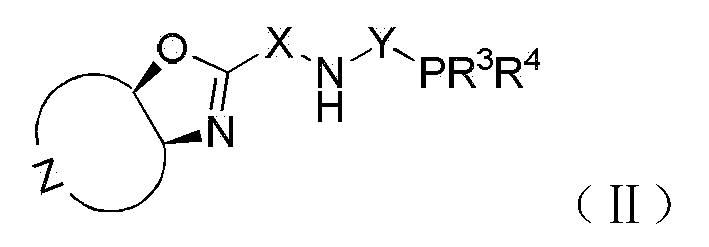
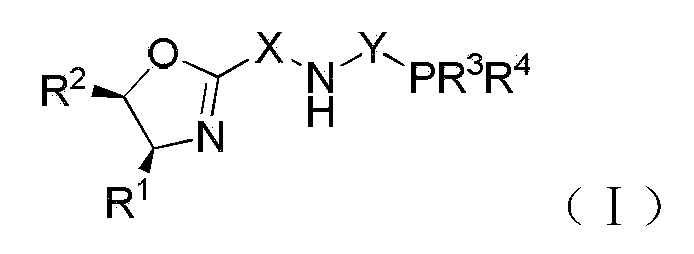Chiral tridentate PNN ligand and application of same in asymmetric hydrogenation
A chemical reaction and chiral technology, applied in the field of chiral tridentate nitrogen phosphine ligands and its application in asymmetric hydrogenation reactions, can solve problems such as complex synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1L21
[0058] The synthesis of embodiment 1L21f-(S)-(R)-amphox
[0059]
[0060] N 2 A hexane solution of n-butyllithium (12.4mL, 1.4M) under protection was added dropwise to (3.6g, 14mmol) of (S)-1 in 20mL of anhydrous diethyl ether solution, keeping the temperature at 0°C and controlling Dropping time was 20 minutes. After the dropwise addition, the reaction solution was stirred at room temperature for 1.5 h, and then a solution of diphenylphosphine chloride (6.2 g, 28 mmol) in 10 mL of diethyl ether was slowly added dropwise. After the dropwise addition was completed, it was refluxed for 4h. Cool the reaction solution to room temperature, put it in an ice-water bath, slowly add a saturated aqueous solution of sodium bicarbonate dropwise, and extract the orange-yellow product with ether, combine the organic phases, wash with water, dry over anhydrous sodium sulfate, and dry in vacuo to obtain an orange oil liquid. After column purification and recrystallization from ethanol,...
Embodiment 2L25
[0072] The synthesis of embodiment 2L25 (R)-amphox
[0073]
[0074] N 2 CH under protection to 2-chloroethylamine hydrochloride (1 g, 8.62 mmol) 2 Cl 2 (20mL) solution was added Boc 2 O (2.07 g, 9.48 mmol) and triethylamine (1.56 mL, 11.2 mmol). After the reaction solution was stirred at room temperature for 24 h, TLC monitored the completion of the reaction and added water to separate the organic phase, and the water phase was separated with CH 2 Cl 2 Extracted three times with anhydrous Na 2 SO 4 After drying and filtration, the solvent was removed under reduced pressure. The target product 2 was purified by column chromatography as a yellow oil with a yield of 97%.
[0075]
[0076] 0°C, N 2 Ph 2 PH (18.6g, 100mmol) was slowly added dropwise into the anhydrous THF solution of t-BuOK (13.5g, 120mmol), after the dropwise addition was completed, it was raised to room temperature and stirred for 40min, then the raw material 2 was slowly added to the reaction so...
Embodiment 3
[0081] The synthesis of embodiment 3 (R)-amphox catalyst
[0082]
[0083] RuHCl(CO)(PPh 3 ) 3 (0.95g, 1mmol) and the ligand L25(R)-amphox (0.42g, 1.05mmol) were put into 7ml of anhydrous and oxygen-free 1,4-dioxane solvent, heated to reflux for 3h and then cooled to room temperature. Diethyl ether was added to the system and the temperature was lowered to -15°C to obtain crystals and filtered. The solid was washed twice with ether and then dried in vacuo to obtain a yellow solid with a yield of 88%, which indicated that the catalyst could be used for catalytic reactions without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com