Preparation method of bio-based copolyester containing modifiable functional group
A copolyester and bio-based technology, which is applied in the fields of polymer materials and chemical engineering, can solve the problems of unfavorable biomedical applications, high biological toxicity, and low atom utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
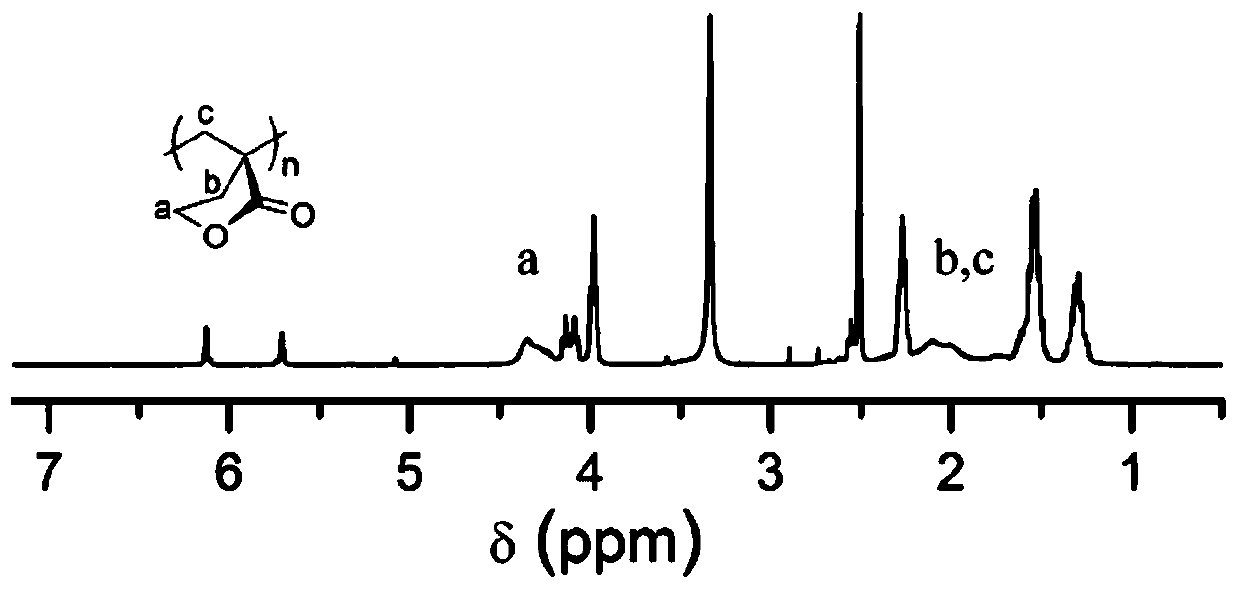
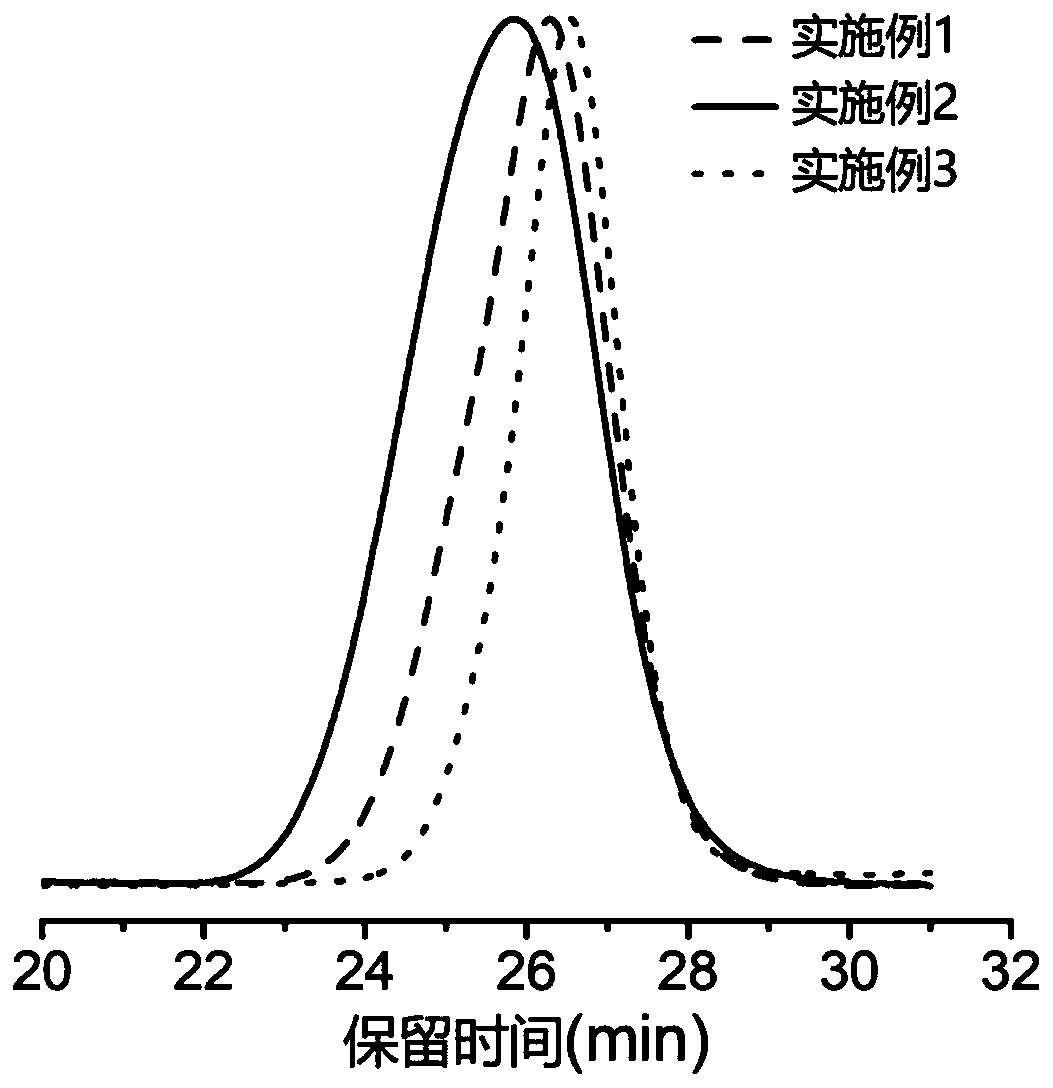
[0044] (0.15mmol, 16.2mg) benzyl alcohol, (0.15mmol, 179.7mg) hexa[tri(dimethylamine) phosphazene]tripolyphosphazene, (0.45mmol, 113.7mg) 1-cyclohexyl-3- (4-Chlorophenyl) urea was dissolved in 6.0mL tetrahydrofuran, placed in a low-temperature cold bath at -50°C and stirred for 10 minutes, and (7.5mmol, 0.66mL) α-methylene-γ-butyrolactone and ( 7.5mmol, 0.84mL) ε-caprolactone was added into the reaction tube at the same time. The reaction was carried out under nitrogen protection for 2 h, and 10 drops of acetic acid was added to terminate the reaction. The reaction mixture was dissolved in 9 mL of chloroform, poured into 60 mL of methanol, centrifuged and precipitated to obtain a polymer, which was characterized by NMR as a ring-opened copolyester, namely PMBL-co-PCL. The number average molecular weight measured by CPC is 10.6kg / mol, the molecular weight distribution is 1.13, and the GPC spectrogram is as follows figure 2 shown. Compared with Comparative Example 1, there i...
Embodiment 2
[0046] (0.05mmol, 5.41mg) benzyl alcohol, (0.05mmol, 59.9mg) hexa[tri(dimethylamine) phosphazene]tripolyphosphazene, (0.15mmol, 32.75mg) 1-cyclohexyl-3- Phenylurea was dissolved in 2.0mL tetrahydrofuran, placed in a low-temperature cold bath at -40°C and stirred for 10 minutes, and (2.5mmol, 0.22mL) α-methylene-γ-butyrolactone and (2.5mmol, 0.28mL ) ε-caprolactone was added to the reaction tube at the same time. The reaction was carried out under nitrogen protection for 1 h, and 10 drops of benzoic acid were added to terminate the reaction. The reaction mixture was dissolved in 3 mL of chloroform, poured into 40 mL of methanol, centrifuged and precipitated to obtain a polymer, which was characterized by nuclear magnetic resonance as a ring-opened copolyester, namely PMBL-co-PCL, and its number-average molecular weight measured by CPC was 12.8 kg / mol, the molecular weight distribution is 1.30, and the GPC spectrum is as follows figure 2 shown.
Embodiment 3
[0048] (0.10mmol, 4.6mg) ethanol, (0.10mmol, 119.8mg) hexa[three (dimethylamine) phosphazene] tripolyphosphazene, (0.20mmol, 57.3mg) 1-cyclohexyl-3-( 4-Trifluoromethylphenyl)urea was dissolved in 4.0ml tetrahydrofuran, placed in -50°C low-temperature cold bath and stirred for 10min, and (5mmol, 0.44mL) α-methylene-γ-butyrolactone and (5mmol, 0.56mL) ε-caprolactone mixed solution was added to the reaction tube. The reaction was carried out under nitrogen protection for 4 h, and 10 drops of acetic acid was added to terminate the reaction. The reaction mixture was dissolved in 4 mL of chloroform, poured into 40 mL of methanol, centrifuged and precipitated to obtain a polymer, which was characterized by NMR as a ring-opened copolymer, namely PMBL-co-PCL. The number average molecular weight recorded by CPC is 9.6kg / mol, and the molecular weight distribution is 1.12, and its GPC spectrum is as follows figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com