Method for directly preparing gamma-valerolactone from acetylpropionic acid and aminic acid
A technology of levulinic acid and valerolactone, applied in directions such as organic chemistry, to achieve the effects of simple and convenient operation process, improved environmental friendliness, and avoided energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~7
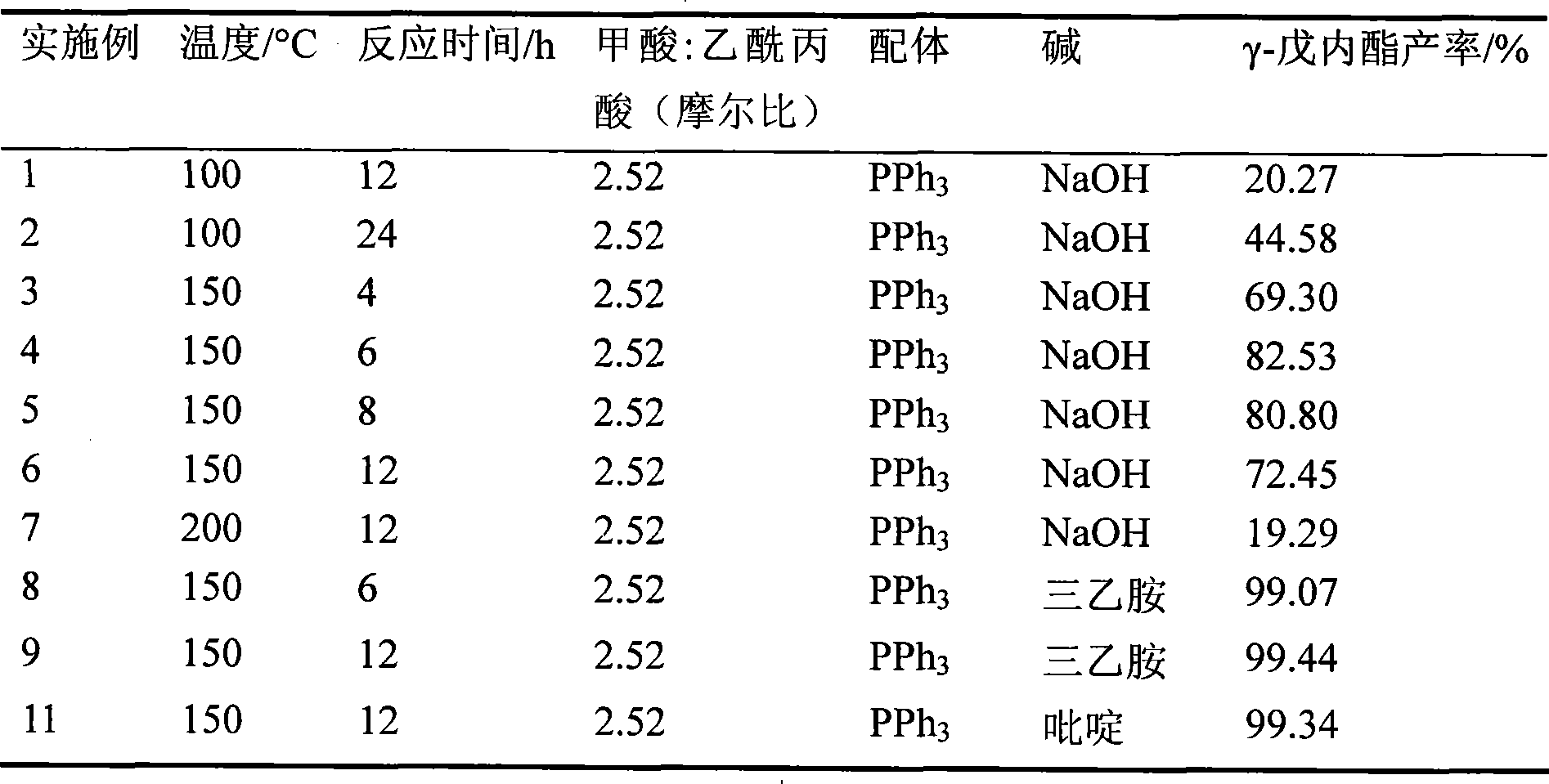
[0025] In the autoclave of 150mL, add 0.2mmol trihydrate ruthenium trichloride, 0.6mmol triphenylphosphine (PPh 3 ), 20mmol sodium hydroxide, 200mmol formic acid and 79.4mmol levulinic acid, sealed and stirred evenly. Heat to 100, 150 or 200°C, keep for 4, 6, 8 or 12 hours, finish the reaction and cool to room temperature, slowly depressurize to atmospheric pressure, open the autoclave, take samples, send to GC-MS for detection, specific experimental temperature, reaction The time and test results are listed in Table 1 with serial numbers 1-7.
Embodiment 8~11
[0027] Add 0.2mmol ruthenium trichloride trihydrate, 0.6mmol triphenylphosphine, 20mmol triethylamine or pyridine, 200mmol formic acid and 79.4mmol levulinic acid into a 150mL autoclave, seal it, and stir well. Heat to 150°C, keep for 6 or 12 hours, finish the reaction and cool to room temperature, slowly depressurize to atmospheric pressure, open the autoclave, take samples at different temperatures and reaction times, send them to GC-MS for detection, and the test results are listed in Table 1 The serial numbers are 8-11.
Embodiment 12~15
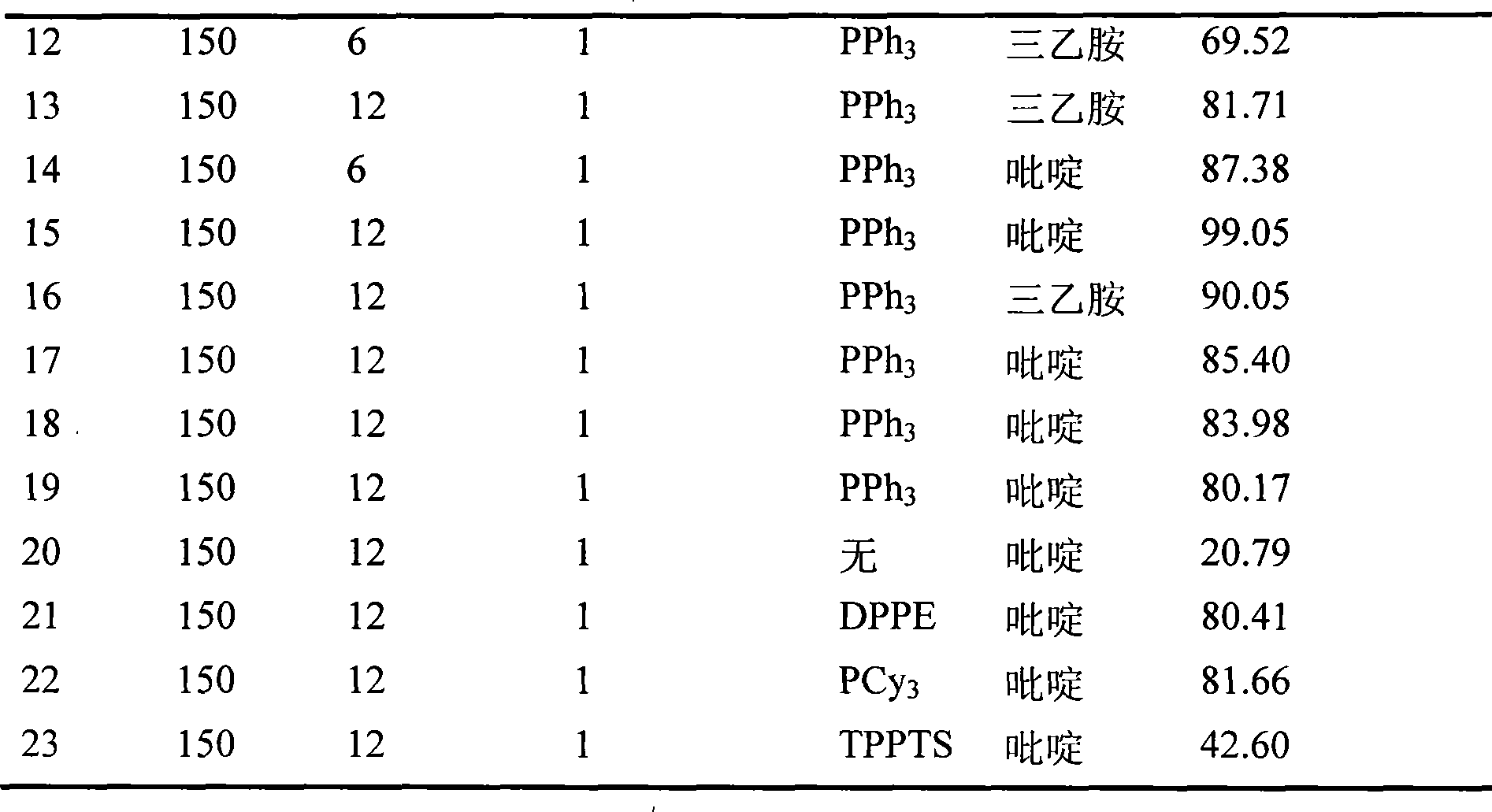
[0029] Add 0.2mmol ruthenium trichloride trihydrate, 0.6mmol triphenylphosphine, 20mmol triethylamine or pyridine, 200mmol formic acid and 200mmol levulinic acid into a 150mL autoclave, seal it, and stir well. Heat to 150°C, keep for 6 or 12 hours, finish the reaction, cool to room temperature, slowly depressurize to atmospheric pressure, open the autoclave, take samples, take samples at different temperatures and reaction times, send them to GC-MS for detection, and the test results are listed in the table The serial number in 1 is 12~15.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com