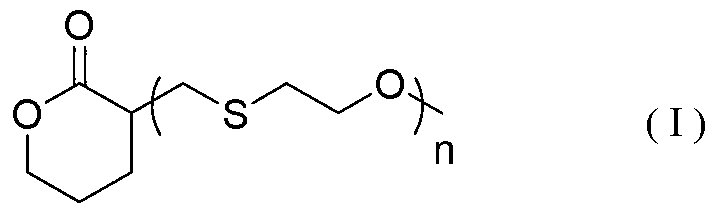
Delta-valerolactone compounds, preparation method and application
A compound, the technology of valerolactone, applied in δ-valerolactone compounds and its preparation method and application field, can solve the problems of accumulation, polyethylene glycol cannot be degraded, aliphatic polyester does not have hydrophilicity, etc. , to achieve the effect of a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The synthesis line:
[0046]
[0047] (1) Add 3.45g sodium metal, 14.6g (0.1mol) diethyl oxalate, and 10.00g (0.1mol) compound 1δ-valerolactone to 100mL ethanol under ice-salt bath conditions, and react in ice bath for 1 hour. React at room temperature for 10 hours, remove the solvent, add water to the residue, wash the aqueous phase with ether, acidify the aqueous phase with hydrochloric acid, extract with dichloromethane, dry and remove the solvent to obtain compound 3 as a yellow liquid;
[0048] (2) Add 0.1 mol NaH with a purity of 60% to 150 mL of anhydrous tetrahydrofuran and stir to form a suspension, then add compound 3 obtained in step 1) dropwise, and react until there are no bubbles to obtain compound 5;
[0049] (3) Continue to feed formaldehyde gas into the above reaction solution, filter after 30 minutes, remove the solvent from the filtrate, and purify to obtain compound 7 as a yellow liquid;
[0050] (4) Dissolve compound 7 obtained in step 3) in d...
Embodiment 2
[0054]
[0055] (1) Add 3.45g sodium metal, 14.6g (0.1mol) diethyl oxalate, and 10.00g (0.1mol) compound 1δ-valerolactone to 100mL ethanol under ice-salt bath conditions, and react in ice bath for 1 hour. React at room temperature for 10 hours, remove the solvent, add water to the residue, wash the aqueous phase with ether, acidify the aqueous phase with hydrochloric acid, extract with dichloromethane, dry and remove the solvent to obtain compound 2 as a yellow liquid;
[0056] (2) Add 0.1 mol NaH with a purity of 60% to 150 mL of anhydrous tetrahydrofuran and stir to form a suspension, then add compound 3 obtained in step 1) dropwise, and react until there are no bubbles to obtain compound 5;
[0057] (3) Continue to feed formaldehyde gas into the above reaction solution, filter after 30 minutes, remove the solvent from the filtrate, and purify to obtain compound 7 as a yellow liquid;
[0058] (4) Dissolve compound 7 obtained in step 3) in dichloromethane, add 6.8 g of com...
Embodiment 3
[0062] The synthetic route of is as follows:
[0063]
[0064] Under anhydrous, oxygen-free, and argon protection conditions, add 0.0011g (0.00001mol) of compound 11 benzyl alcohol to 0.292g (0.001mol) of compound 10, stir well and add 0.0069g of catalyst 1,5,7-triaza Bicyclo[4.4.0]dec-5-ene (TBD), stirred for 1 day, added 20mL of dichloromethane and 0.1g of benzoic acid, and evaporated to remove the solvent; the product was dissolved in 10mL of methanol and placed in a 1000Da dialysis bag. Dialyzed with methanol for 2 days to obtain 0.42 mmol of light yellow oily liquid polyester with a yield of 42%.
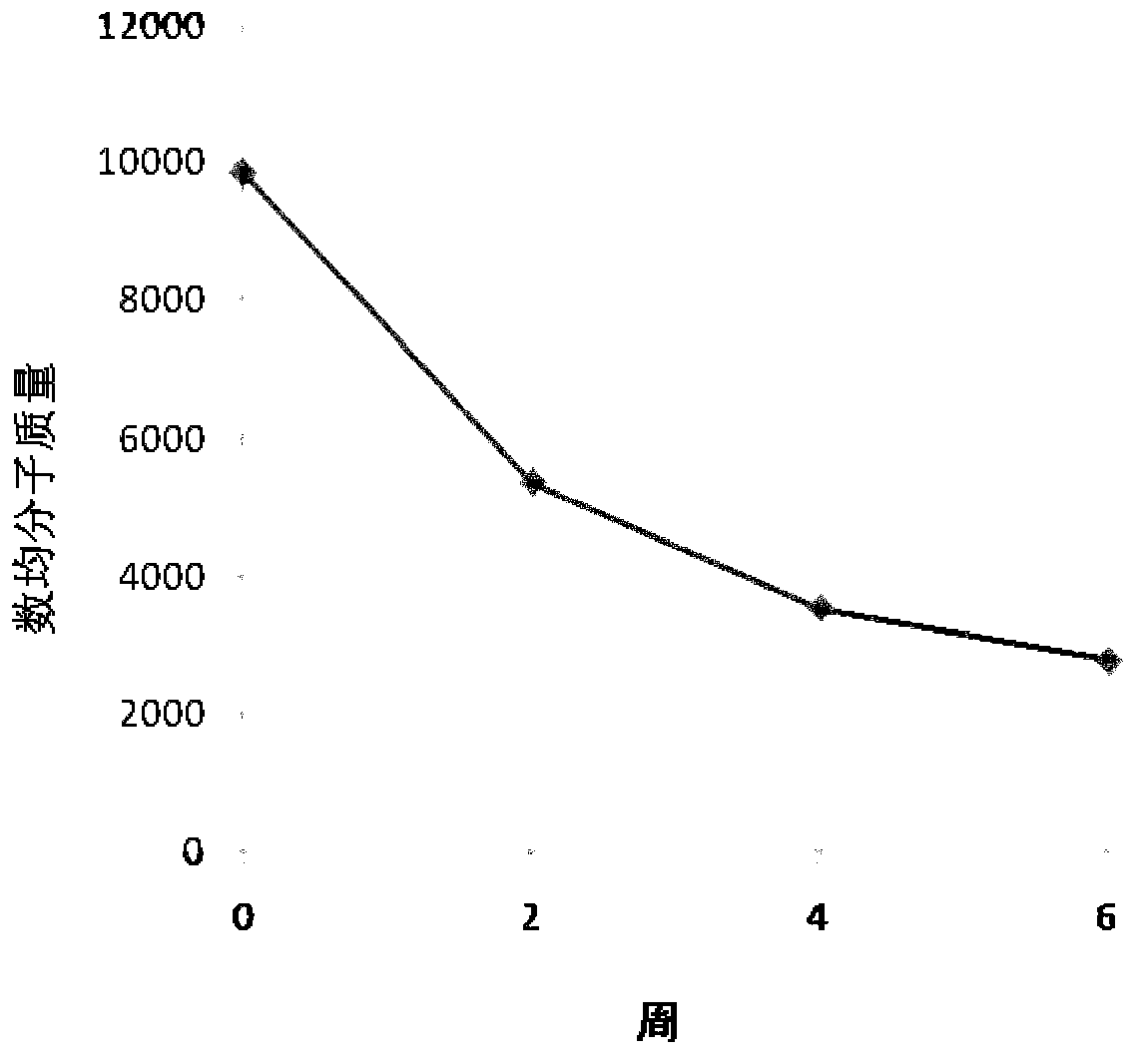
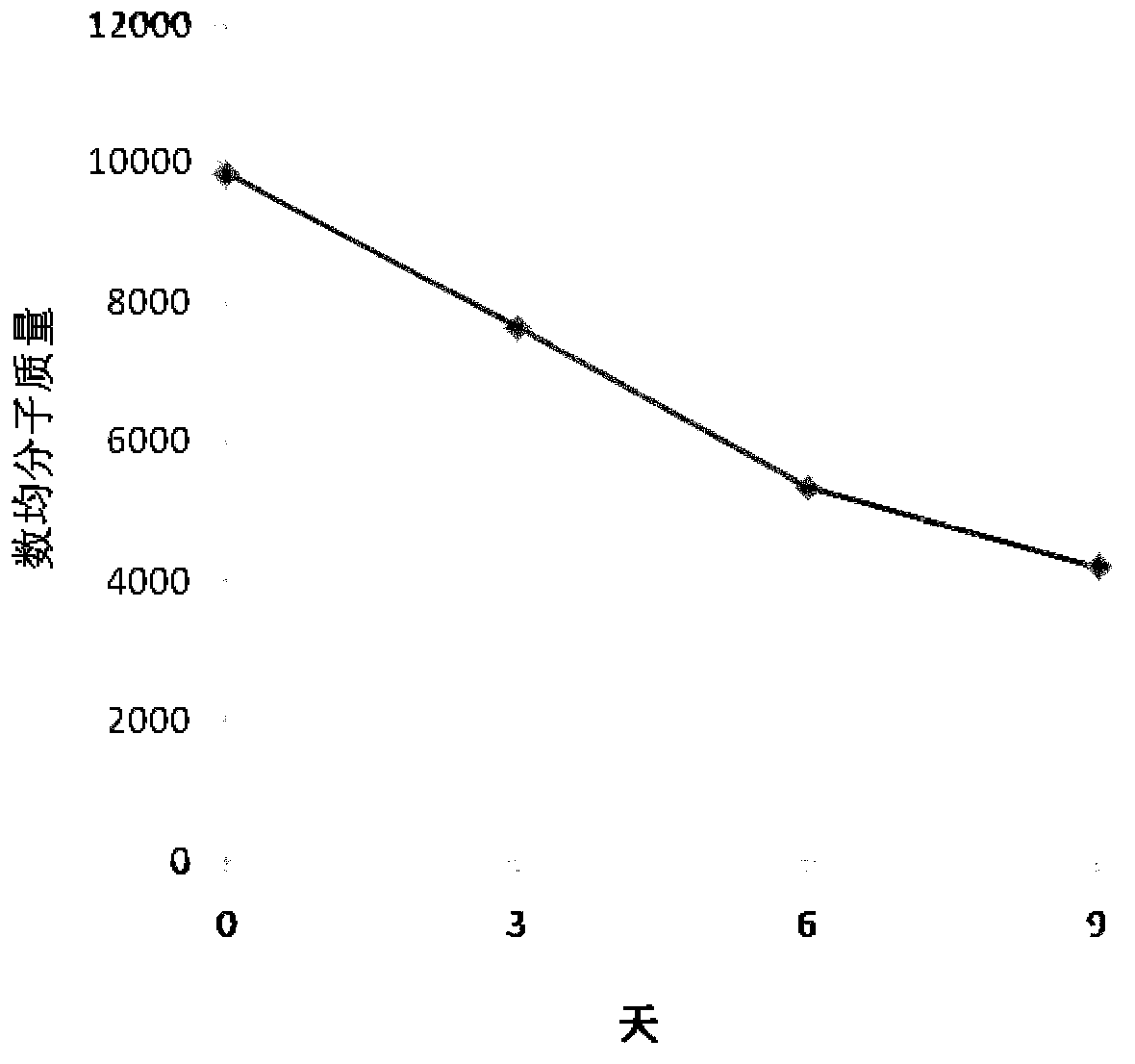
[0065] The physical parameters of the yellowish oily liquid polyester: M n =6557, M w =7444, PDI=1.13.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com