Method for preparing gamma-valerolactone by acetylpropionic acid catalytic hydrogenation
A technology of levulinic acid and valerolactone, applied in chemical instruments and methods, molecular sieve catalysts, physical/chemical process catalysts, etc., can solve the problems of difficulty in adapting to large-scale industrial production, harsh catalyst reaction conditions, and rapid decline in catalyst activity, etc. problems, achieve high industrial application value, improve economy and safety, and achieve high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) Preparation of catalyst
[0048] Weigh 0.05g RuCl into a 100mL beaker 3 ·3H 2 O, dissolved with 8g of secondary water, shake well, add carrier γ-Al to the solution 2 o 3 6g, mixed evenly, soaked at room temperature for 8h, dried at 80°C for 12h, calcined at 300°C in an air atmosphere, and then reduced in a hydrogen atmosphere kettle at 130°C for 2h to obtain the ruthenium-based catalyst provided by the present invention (the Ru loading capacity is 0.32%).
[0049] (2) Catalytic performance test
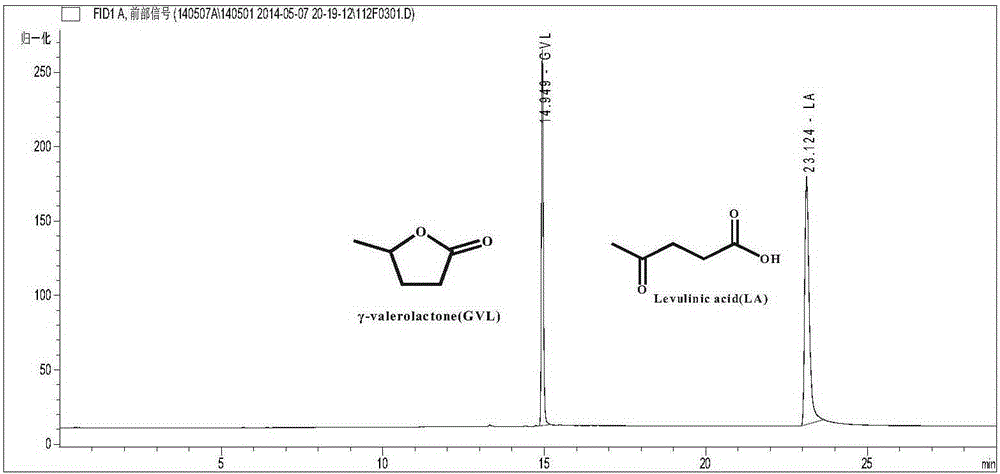
[0050] Add 10g of water as a solvent, 8g of levulinic acid and 0.10g of the above catalyst in the autoclave, seal the autoclave, fill it with 4MPa hydrogen, react at 130°C for 3h, cool to room temperature after the reaction, and slowly depressurize to the atmosphere Pressure, open the autoclave to sample, GC detection, LA conversion rate 30.5%, GVL selectivity 99.9%.
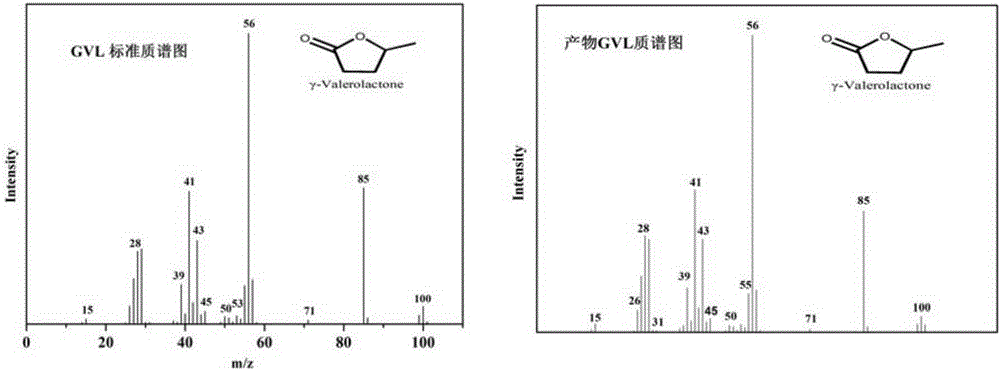
[0051] Wherein, the structure of the resulting product γ-valerolactone (GVL) is confirmed by GC-MS da...
Embodiment 2
[0053] (1) Preparation of catalyst
[0054] Weigh 0.05g RuCl into a 100mL beaker 3 ·3H 2 O, dissolve with 8g of secondary water, shake well, add carrier ZSM-56g to the solution, mix evenly, impregnate at room temperature for 8h, dry at 80°C for 12h, roast at 300°C in an air atmosphere, and then reduce in a hydrogen atmosphere kettle at 130°C for 2h , the ruthenium-based catalyst provided by the present invention (Ru loading is 0.32%) can be obtained.
[0055] (2) Catalytic performance test
[0056] Add 10g of water as a solvent, 8g of levulinic acid and 0.10g of the above catalyst in the autoclave, seal the autoclave, fill it with 4MPa hydrogen, react at 130°C for 3h, cool to room temperature after the reaction, and slowly depressurize to the atmosphere Pressure, open the autoclave to sample, GC detection, LA conversion rate 45.5%, GVL selectivity 99.9%.
Embodiment 3
[0058] (1) Preparation of catalyst
[0059] Weigh 0.05g RuCl into a 100mL beaker 3 ·3H 2 O, dissolve with 8g of secondary water, shake well, add 6g of carrier activated carbon to the solution, mix evenly, impregnate at room temperature for 8h, dry at 80°C for 12h, and reduce in a hydrogen atmosphere kettle at 130°C for 2h to obtain the ruthenium provided by the invention based catalyst (0.32% Ru loading).
[0060] (2) Catalytic performance test
[0061] Add 10g of water as a solvent, 8g of levulinic acid and 0.10g of the above catalyst in the autoclave, seal the autoclave, fill it with 4MPa hydrogen, react at 130°C for 2h, cool to room temperature after the reaction, and slowly depressurize to the atmosphere Pressure, open the autoclave to sample, GC detection, LA conversion rate 90.5%, GVL selectivity 99.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com