Amine imines zinc catalyst and preparation method and use thereof
A technology of amine imine zinc and catalyst, applied in the direction of zinc organic compounds, etc., can solve the problems of long reaction time and low reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
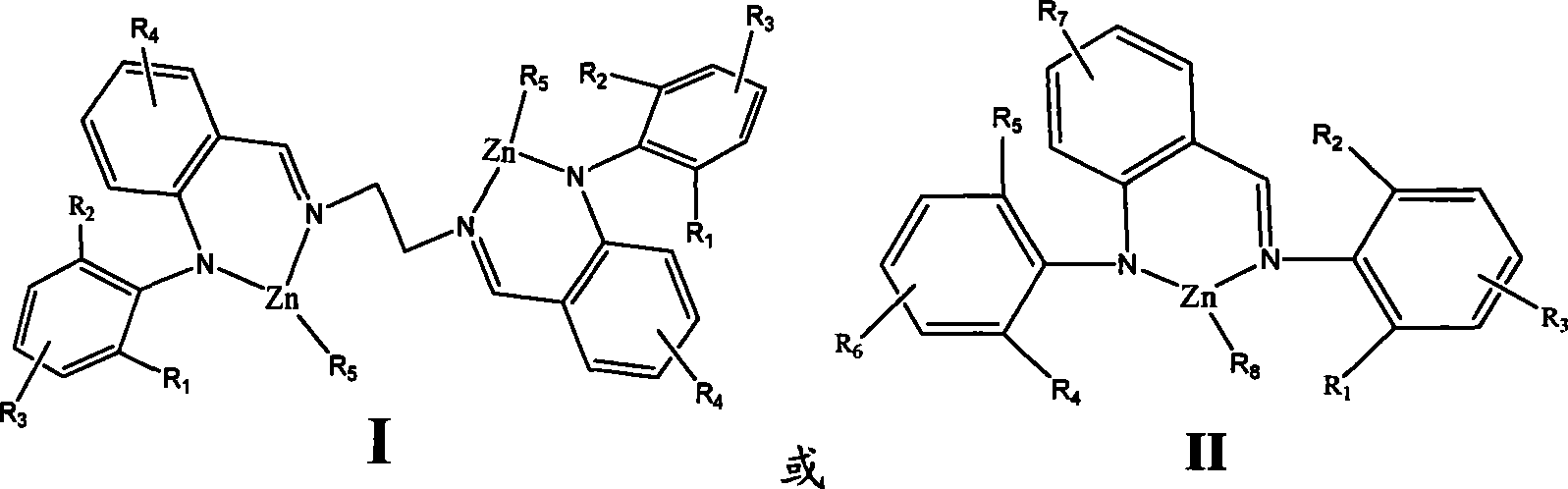
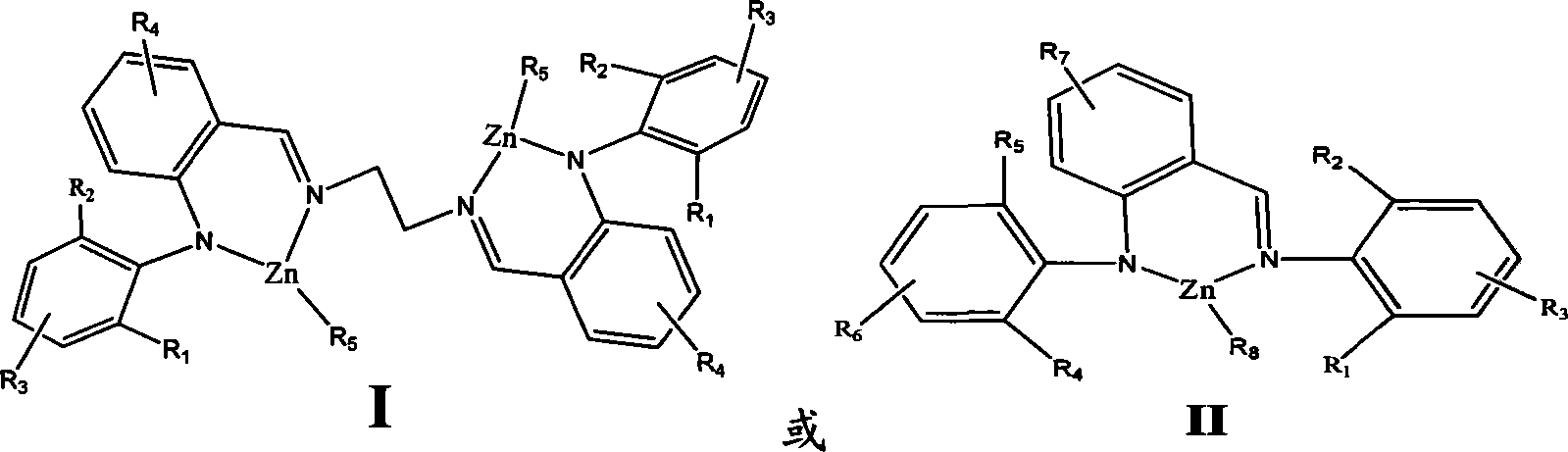
[0029] Example 1 (2-phenyliminomethylphenyl)-anilinoethylzinc
[0030] This compound can be prepared by the following two methods.
[0031] method one:
[0032] Under a nitrogen atmosphere, 0.272 g of (2-phenyliminomethylphenyl) aniline was dissolved in 30 mL of anhydrous tetrahydrofuran, and an equivalent amount of n-butyllithium was added at -78°C, and stirred at room temperature for 2 hours. Add an equivalent amount of ethyl zinc chloride compound in tetrahydrofuran at -78°C, stir for 10 to 12 hours, remove the solvent, and wash the crude product with CH 2 Cl 2 and recrystallized from hexane. 0.251 g of pure product was obtained, with a yield of 76.4%.
[0033] Method Two:
[0034] Under a nitrogen atmosphere, dissolve 0.272 g of (2-phenyliminomethylphenyl) aniline in 20 mL of toluene, add an equivalent amount of diethylzinc at -78 ° C, rise to room temperature and stir overnight, and remove the solvent. Crude product with CH 2 Cl 2 and recrystallized from hexane. ...
Embodiment 2
[0037] Example 2 (2-2,6-Diisopropylphenyliminomethylphenyl)-2,6-Diisopropylanilinoethylzinc
[0038] Under a nitrogen atmosphere, dissolve 0.441 g of (2-2,6-diisopropylphenyliminomethylphenyl)-2,6-diisopropylaniline in 20 mL of toluene at -78°C Add an equivalent amount of diethylzinc, rise to room temperature and stir overnight, remove the solvent, and use CH 2 Cl 2 and recrystallized from hexane. 0.490 g of pure product was obtained, and the yield was 91.7%.
[0039] Elemental analysis results: C74.23; H8.32; N5.20.
Embodiment 3
[0040] Example 3 (2-2,6-dimethylphenyliminomethylphenyl)-2,6-dimethylanilinoethylzinc
[0041] Under a nitrogen atmosphere, dissolve 0.328 g of (2-2,6-dimethylphenyliminomethylphenyl)-2,6-dimethylaniline in 20 mL of toluene, and add equivalent diethylzinc, rose to room temperature and stirred overnight, removed the solvent, and the crude product was washed with CH 2 Cl 2 and recrystallized from hexane. 0.387 g of pure product was obtained with a yield of 91.7%.
[0042] Elemental analysis results: C71.20; H6.67; N6.61.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com