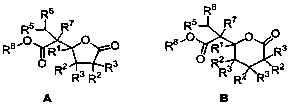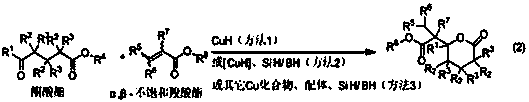Synthetic method of gamma-alkyl oxyacyl methyl-gamma-butyrolactone and delta- alkyl oxyacyl methyl-delta-valerolactone
A technology of acyloxyacylmethyl and synthesis method, applied in the direction of organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The following reaction was carried out by method 1 to obtain the product.
[0058] The following reaction was carried out by method 1 to obtain the product.
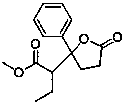
[0059] Add [CuHPPh 3 ] 6 (4.890 g, 15 mmol CuH), toluene (30 mL), stirred to dissolve, cooled to -30°C, and maintained at this temperature in subsequent reactions. A solution of methyl 4-oxo-4-phenylbutyrate (2.300 g, 12.0 mmol) and methyl 2-butenoate (1.6 mL, 15.1 mmol) in toluene (10 mL) was added dropwise to the above solution, After about 20 min of dripping, the stirring reaction was continued for 0.5 h. Warm to room temperature, stir for 0.5 h, then add 20 mL of saturated NH 4 Cl aqueous solution, and continue stirring for 0.5 h. Stand to separate phases. The aqueous phase (3×10 mL) was extracted with toluene, and the combined organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography to obtain γ-phenyl-γ-(1-methyl oxyacyl)p...
Embodiment 2
[0062] The following reaction was carried out by method 2 to obtain the product.
[0063] Vacuumize the dried reaction bottle, inject nitrogen, repeat three times, add [CuHPPh 3 ] 6 (16 mg, 0.049 mmol CuH) and toluene (1 mL), stirred at 25°C for 2 min to make [CuHPPh 3 ] 6 All melted. Add poly(methylhydrogensiloxane) (120 mL, 2.00 mmol SiH) and methyl 4-oxo-4-phenylbutyrate (0.193 g, 1.00 mmol) to the above solution, stir for 2 min and then add acrylic acid A solution of methyl ester (137 mL, 0.152 mmol) in toluene (2 mL). After stirring for 2.0 h, add 2 mL of 1.5 mol / L NH 4 Methanol-water solution (methanol:water = 3:1) of F, stirred for 0.5 h, then stood still for phase separation, the aqueous phase filtrate was extracted with dichloromethane (3×10 mL), and the combined organic phase was washed with saturated brine, After drying over anhydrous sodium sulfate and concentration, the product γ-phenyl-γ-(1-methoxyacyl)ethyl-γ-butyrolactone (0.203 g, yield 82%) was obtaine...
Embodiment 3
[0067] Add CuF (5.0 mg, 0.060 mmol), 1,1'-bis(di-phenylphosphino)ferrocene, (33.2 mg, 0.060 mmol), ethyl acetate to a dry reaction flask under nitrogen atmosphere (3 mL), stirred at 40°C for 30 min. Add diphenylsilane (2.22 mL, 24.0 mmol SiH) to the above solution, stir for 30 min, then add 4-oxo-4-phenylbutyric acid methyl ester (2.30 g, 12 mmol), drop into methyl acrylate Ethyl acetate solution (10 mL) of ester (2.20 mL, 24 mmol) was dropped in about 10 min and stirred for 5 h. Add 30 mL of 1.5 mol / L NH to the reaction mixture 4 Methanol-water solution of F (methanol: water = 3:1), stirred for 0.5 h and allowed to stand for phase separation. The aqueous phase was extracted with ethyl acetate (3 x 30 mL). The combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography to obtain the product γ-phenyl-γ-(1-methoxyacyl)ethyl-γ-butyrolactone (1.63 g, yield 55%).
[0068]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com