Preparation method for human immunoglobulin for intravenous injection
A technology of human immunoglobulin and intravenous injection, applied in the field of preparation of human immunoglobulin by intravenous injection, can solve the problem that the purity can only reach 95%, and achieve the advantages of shortening the production cycle, improving the product yield and improving the product yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
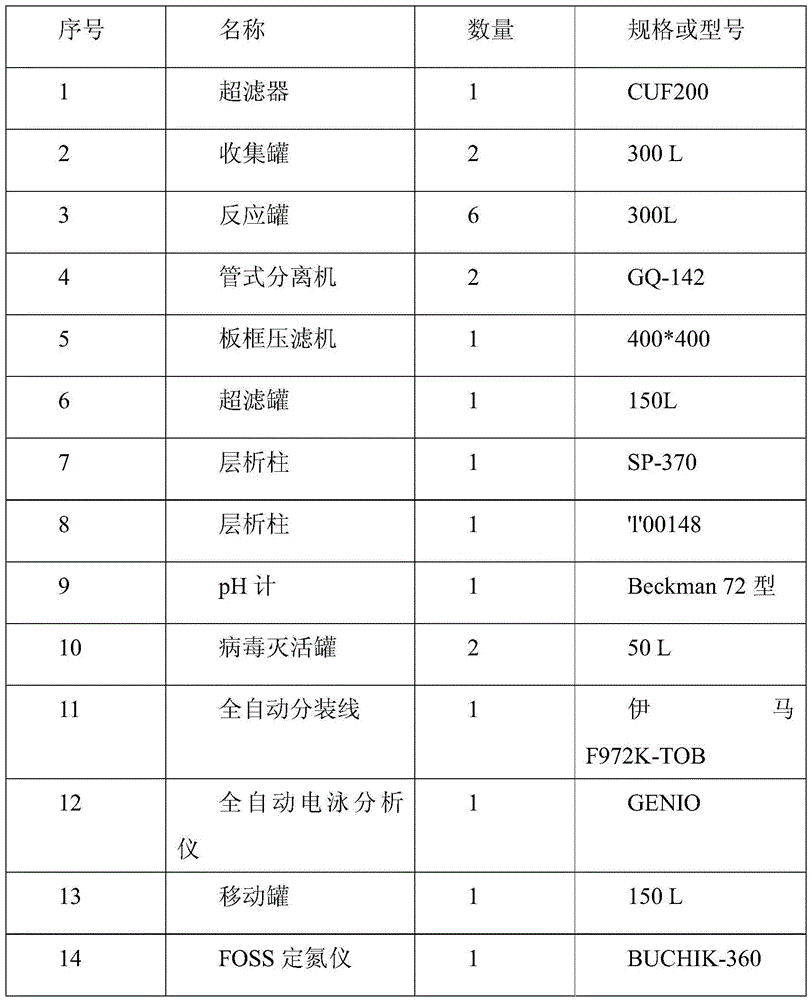
[0039] The plasma source of the present invention is human plasma conforming to the provisions of "Plasma for the Production of Biological Products" in the Pharmacopoeia of the People's Republic of China (2010 Edition).
[0040]It is the two-step filter press method of the present invention: get respectively 100 liters, 200 liters, 500 liters and 1000 liters of qualified human plasma, taking 100 liters of human plasma as an example.
[0041] (1) Centrifuge the human plasma that has passed the quarantine. During centrifugation, the centrifugation speed is 3L / min / unit, and the temperature of the liquid is controlled at 2°C; separate the cryoprecipitate, collect the protein solution into the reaction tank, and control the temperature of the protein solution at At 1°C, add pH4.0 acetate buffer at a flow rate of 0.1L / min to adjust the pH of the protein solution to 7.2; add -17°C 95% ethanol at a flow rate of 60L / h to make the final concentration of ethanol volume ratio to 8%, and co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com