Magnesium barium thioaluminate and related phosphor materials
a phosphor material and magnesium barium thioaluminate technology, applied in the field of magnesium barium thioaluminate and related phosphor materials, can solve the problems of increased performance, degrade the properties of phosphor materials, and insufficient accuracy of shadow mask techniques
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example ii
[0067] A series of magnesium barium thioaluminate thin film phosphors materials were prepared by blending powders of aluminum sulphide, barium sulphide, magnesium sulphide and europium sulphide in the desired ratios and making pressed pellets of the blended powders. In the series of phosphor materials, the fraction "a" of barium replaced by magnesium in the formula Mg.sub.aBa.sub.1-aAL.sub.2 S.sub.4:Eu was varied in increments of 0.1 over the range of a=0 to a=0.5. All of the phosphor materials had a nominal concentration of europium corresponding to 3 atomic percent of the sum of the magnesium and barium concentrations.
[0068] The pellets were placed in an alumina boat and fired in a nitrogen atmosphere using a belt furnace, using a temperature profile such that the films were subject to a nominal peak temperature of 900.degree. C. for about 7 minutes. The actual sample temperature may have been lower than 900.degree. C. because of the thermal capacity of the alumina boat.
[0069] The...
example iii
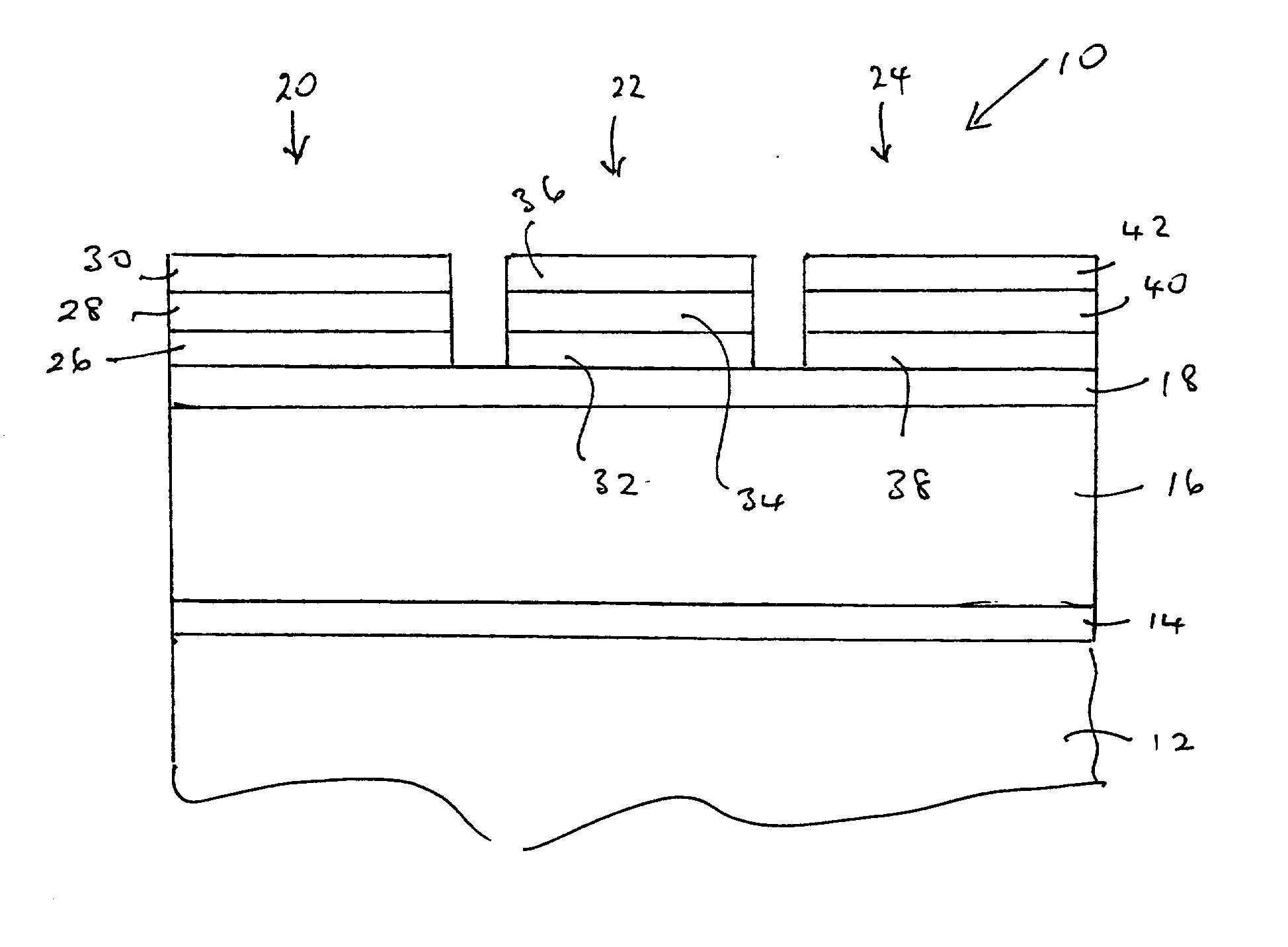
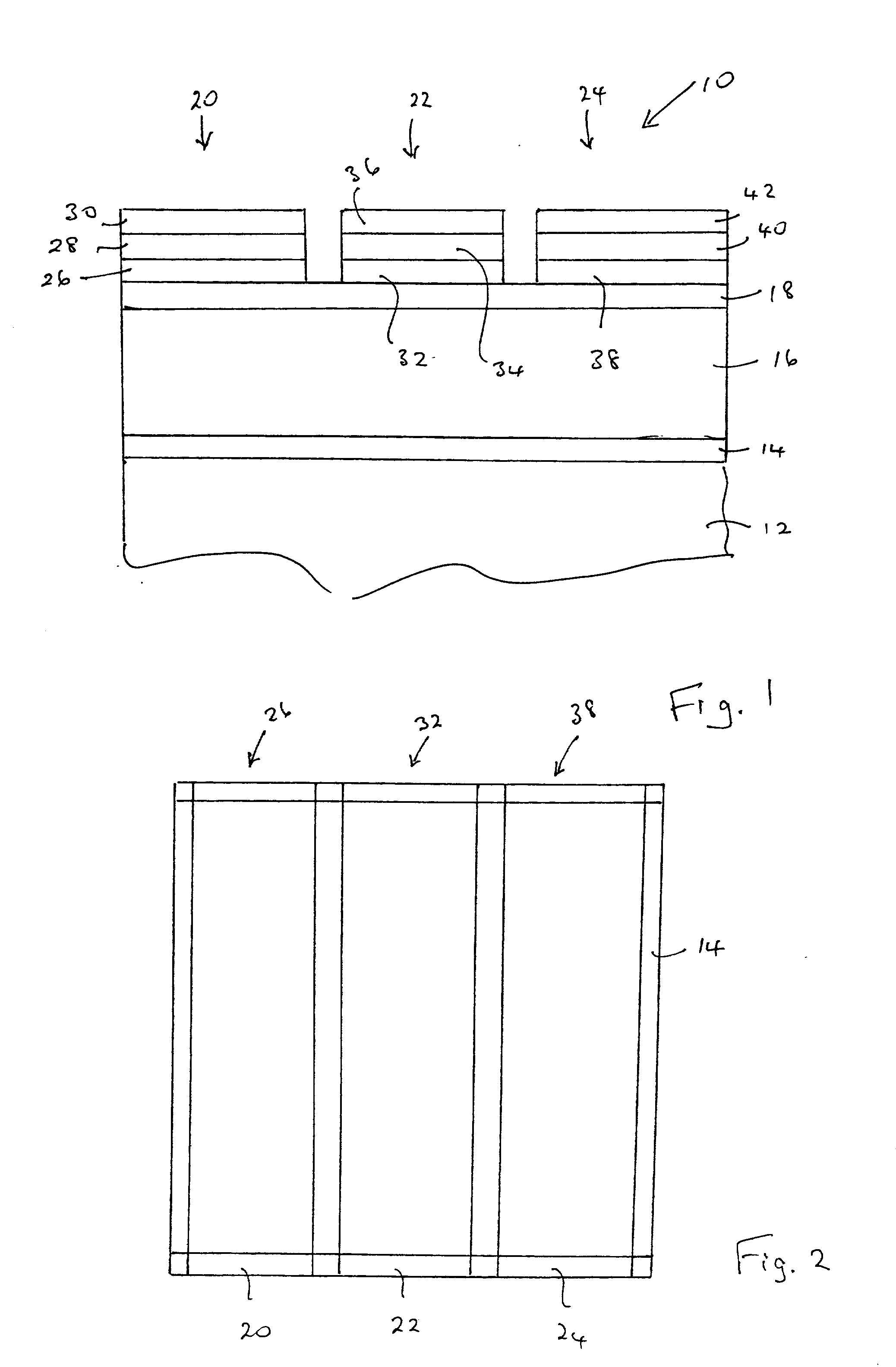
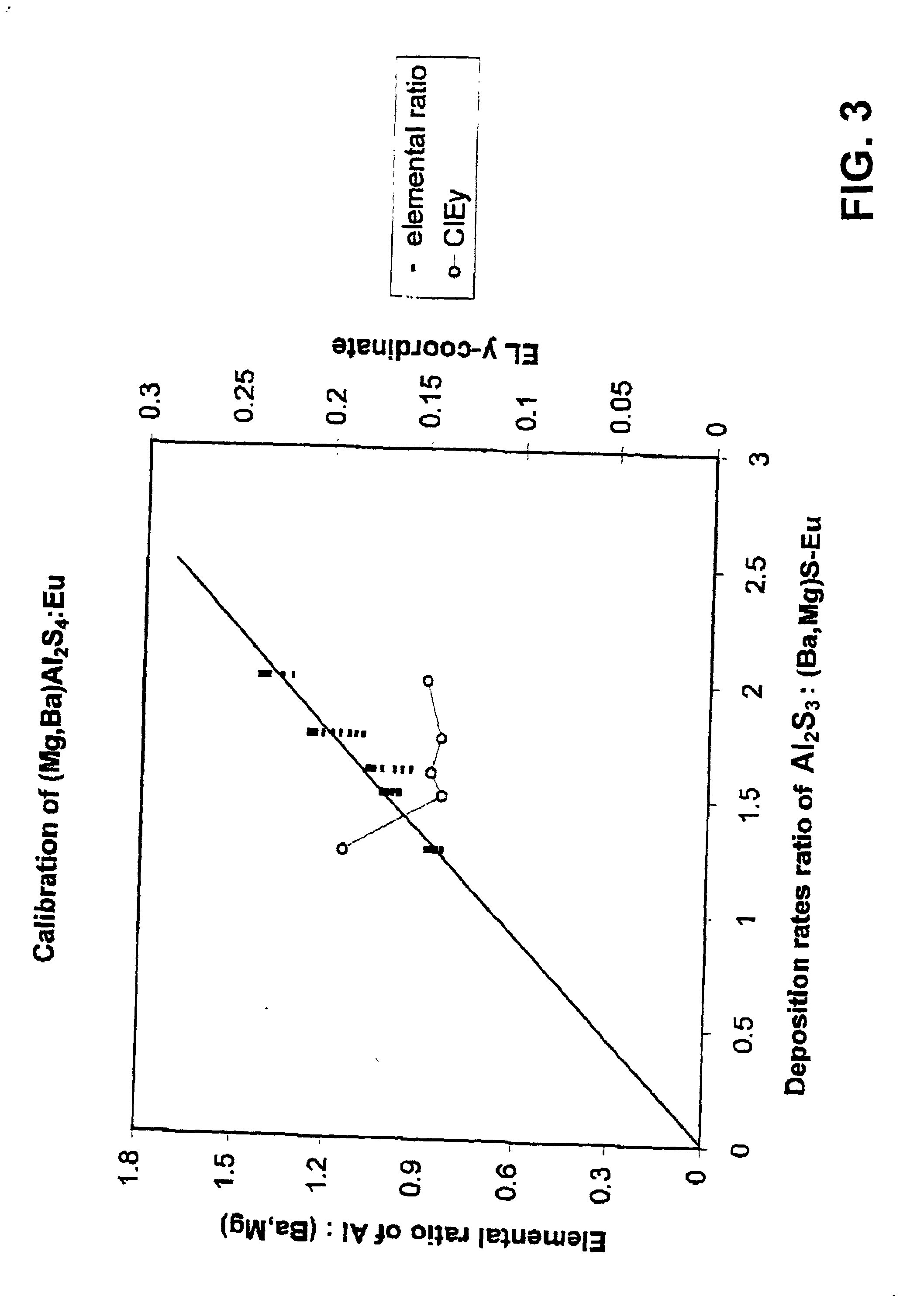
[0076] Magnesium barium thioaluminate materials of the formula Mg.sub.aBa.sub.1-aAl.sub.2S.sub.4:Eu, with a nominal value of a=0.5 and a europium concentration equal to 3 atomic percent of the sum of the magnesium and barium concentrations, were deposited as thin films on thick film dielectric structures. The deposition method used was dual source electron beam evaporation, in which one source was a pressed pellet of aluminum sulphide (Al.sub.2S.sub.3) and the other source was a pressed pellet consisting of a mixture of barium sulphide, magnesium sulphide and europium sulphide. The phosphor films were annealed at a nominal temperature of 850.degree. C. under nitrogen.
[0077] It should be noted that the stated composition for the materials in this example is for the source materials, and the composition of the deposited films may vary from these compositions.
[0078] The electroluminescent emission of the resultant phosphor showed the same blue shift with respect to material not contain...
example iv
[0080] The procedure of Example III was repeated, except that europium oxide (Eu.sub.2O.sub.3) was used instead of europium sulphide. The remaining compounds were aluminum sulphide, barium sulphide and magnesium sulphide, as in Example III. The nominal value of a in the formula of Example III was 0.5. The europium concentration was 3 atomic percent of the sum of the magnesium and barium concentrations.
[0081] It was found that the resultant phosphor had the same characteristics as the phosphor of Example III that had been formed using europium sulphide. It is therefore believed that europium sulphide may be replaced in whole or in part with europium oxide for europium concentrations of up to 3 atomic percent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com