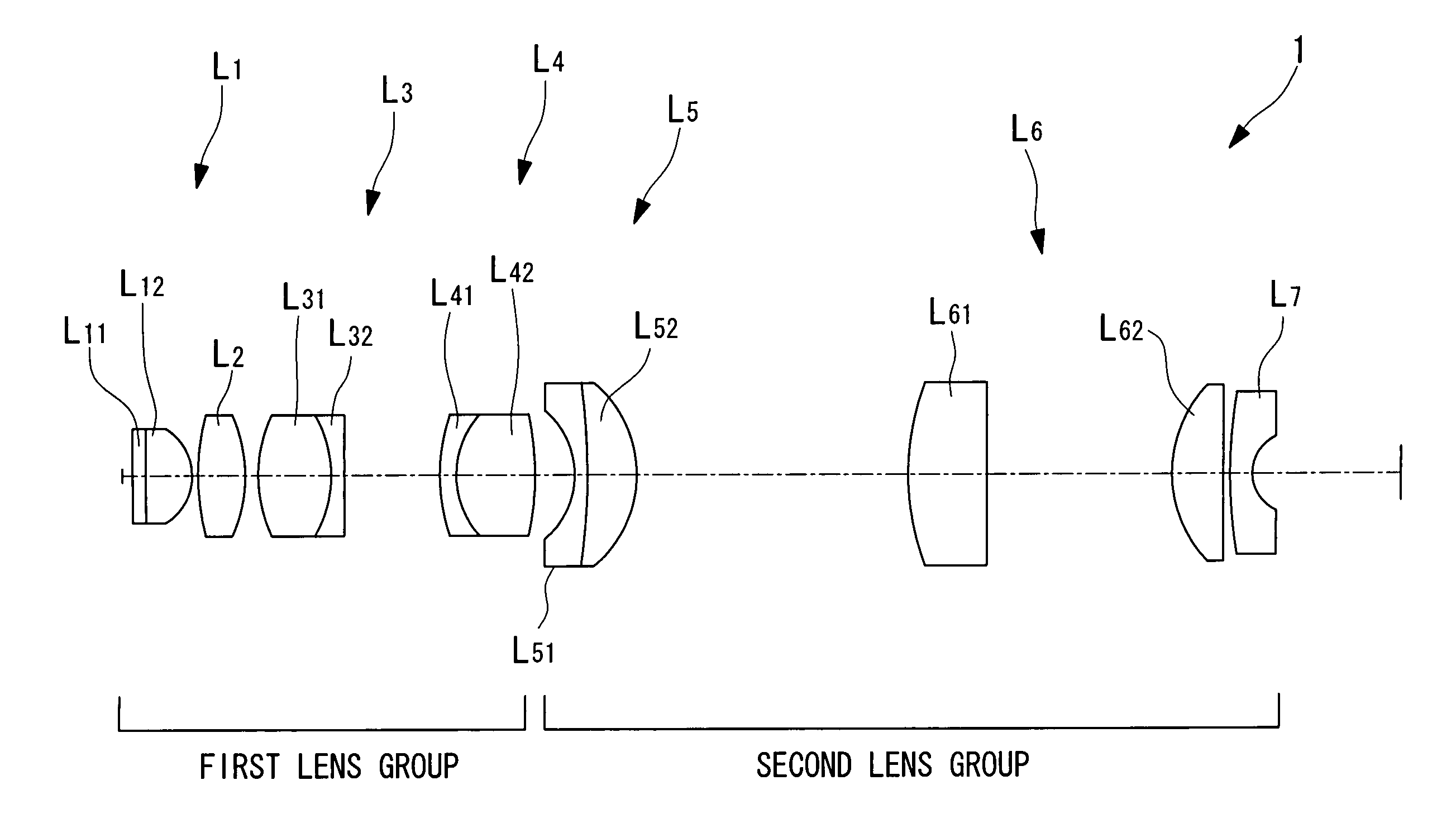Patents
Literature
44results about How to "Minimal invasiveness" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Robotic total/partial knee arthroplastics
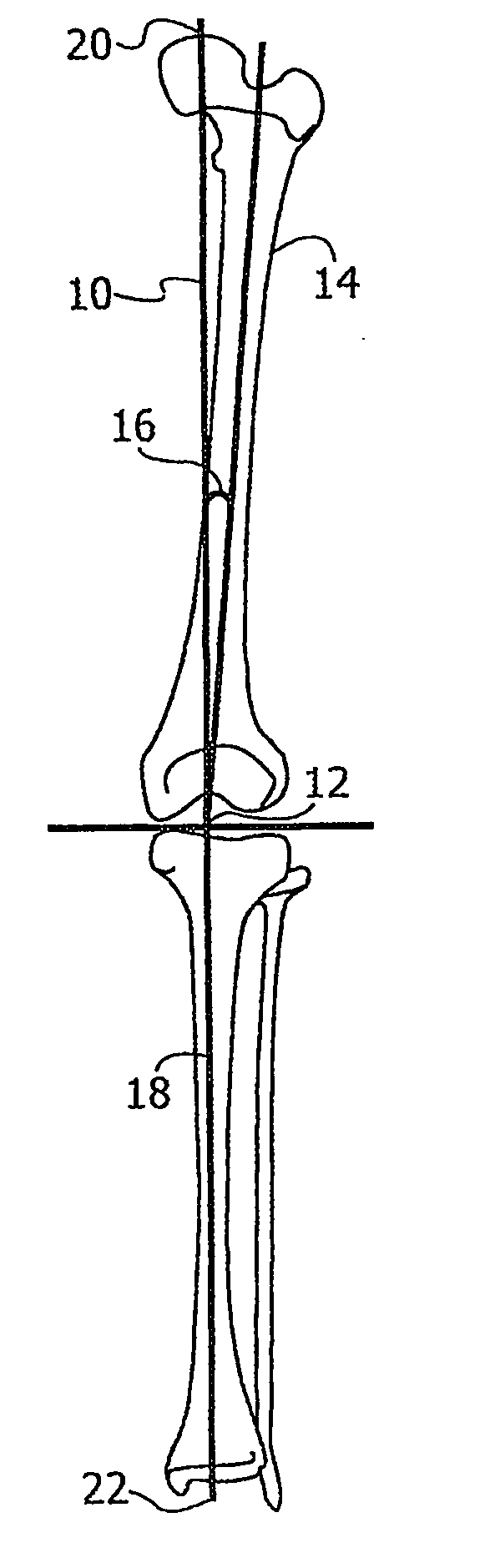
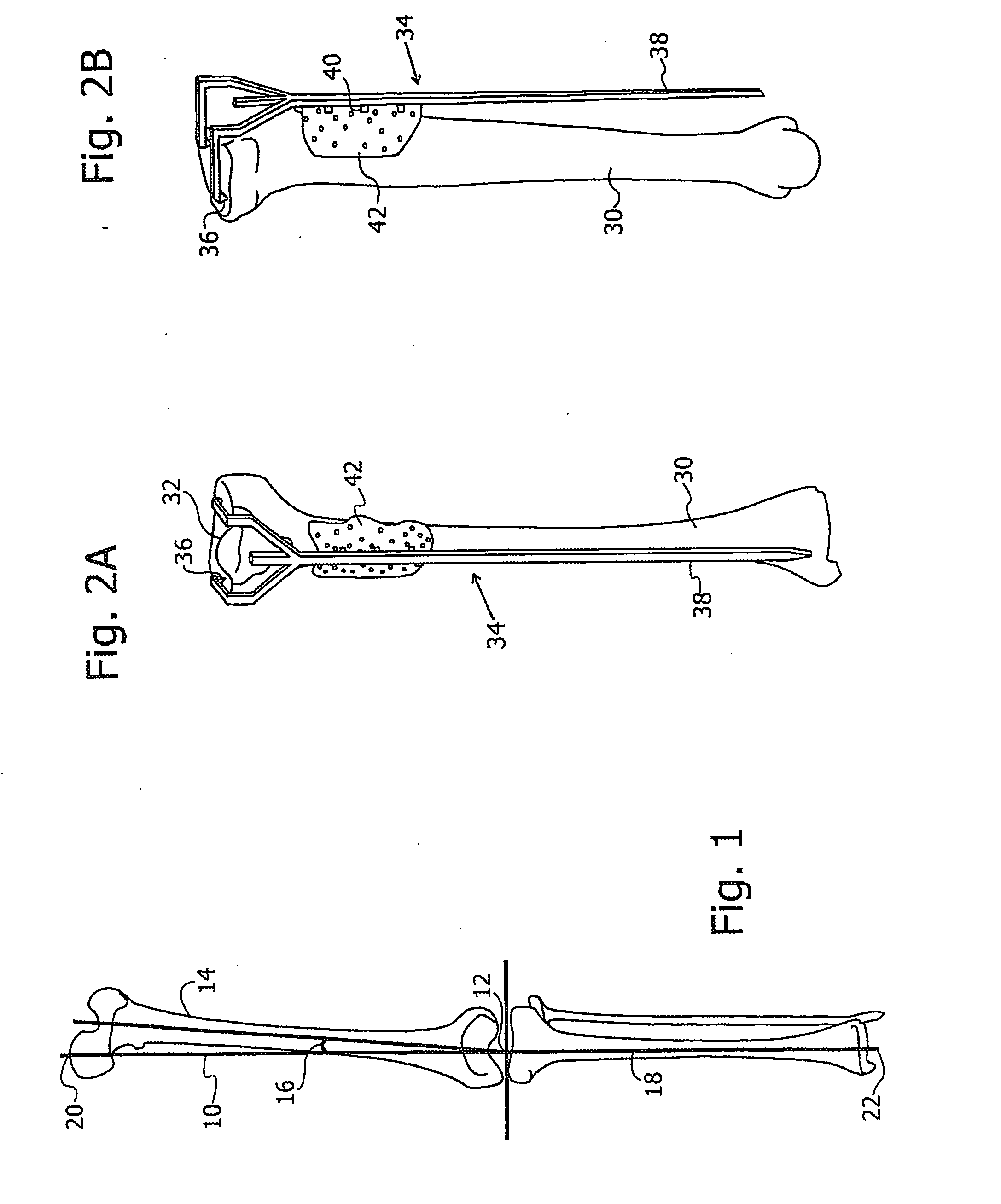
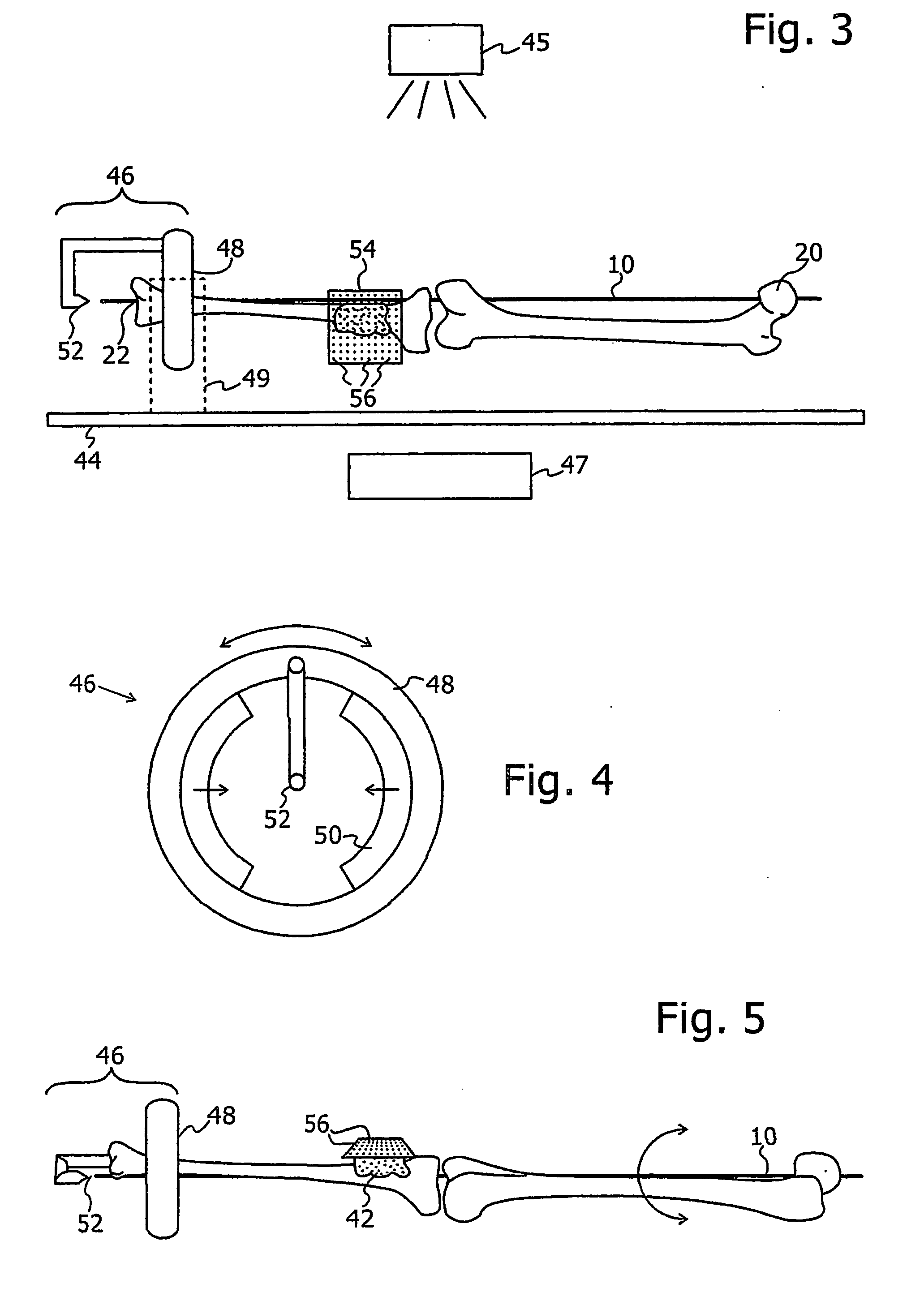
ActiveUS20070100258A1Improve accuracyReduce responsibilityPerson identificationMaterial analysis by optical meansTibiaSurgical robot
A system and method for knee arthroplasty procedures, using a novel leg rotation fixture to enable the leg mechanical axis, the tibia and the femur to be mutually disposed such that the load bearing, mechanical axis of the leg runs through the center of the knee joint. A measurement gauge is provided for mounting on the tibia and for aligning a baseplate in a known position on the tibia. This baseplate supports an X-ray target plate in a known position relative to the tibia, used in determining the mechanical axis, and an optional surgical robot, used to perform tibial and femoral cuts. The position of the femur relative to the robot may be determined from X-ray imaging of the pelvic region after attachment to the baseplate of an additional target extending to the pelvic region. The system enables improvement in the accuracy of knee arthroplasty procedures.
Owner:MAZOR ROBOTICS
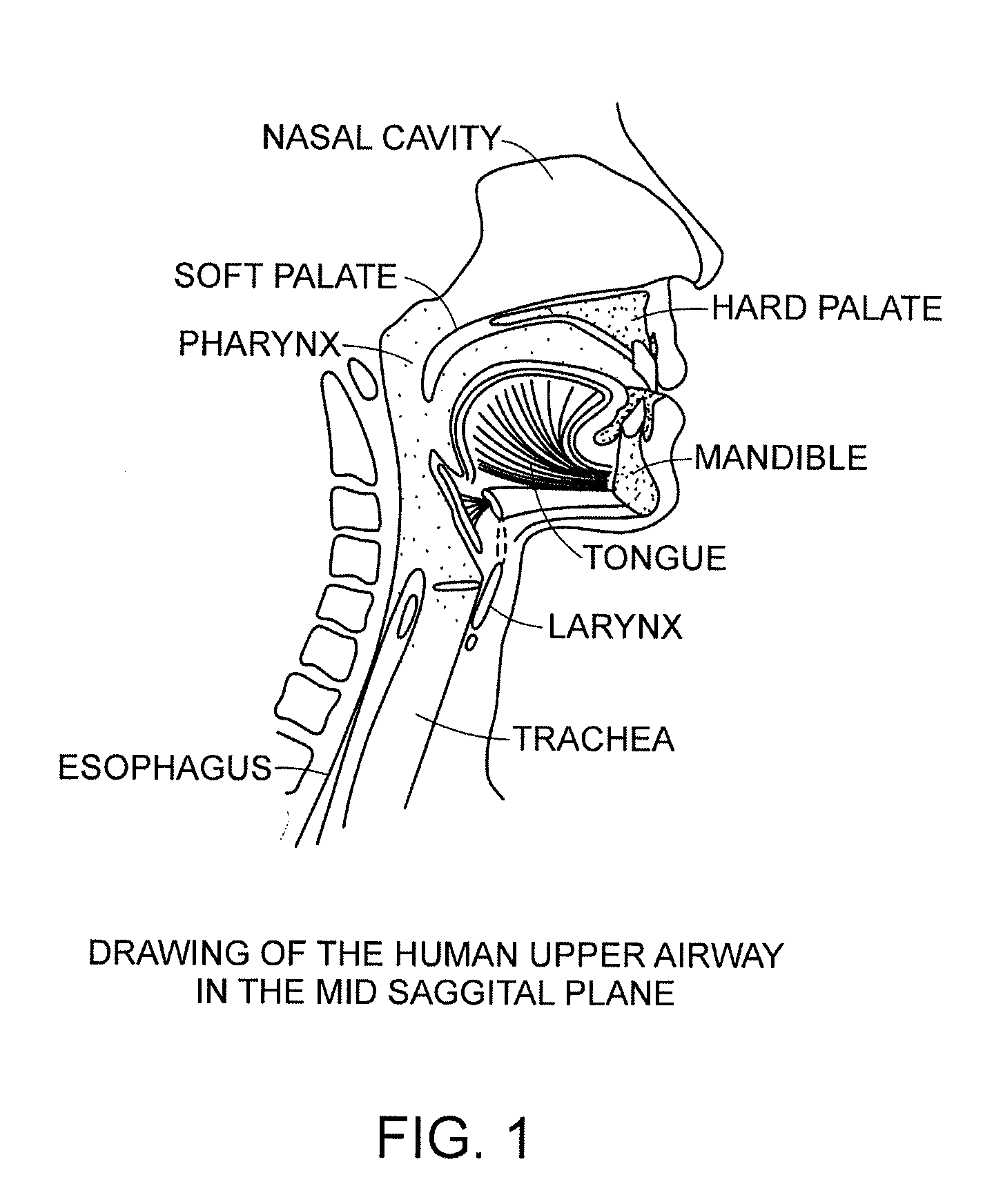
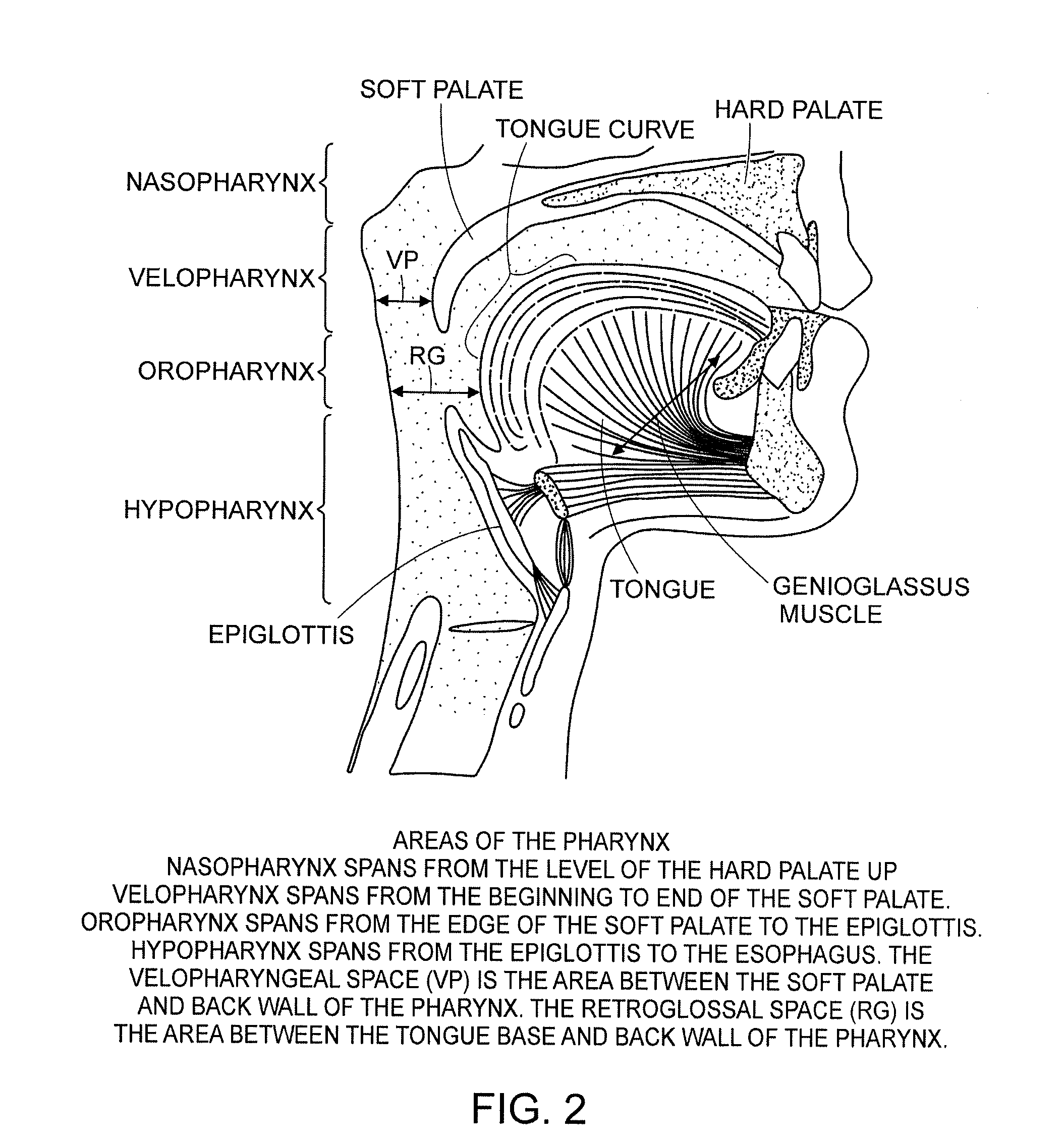
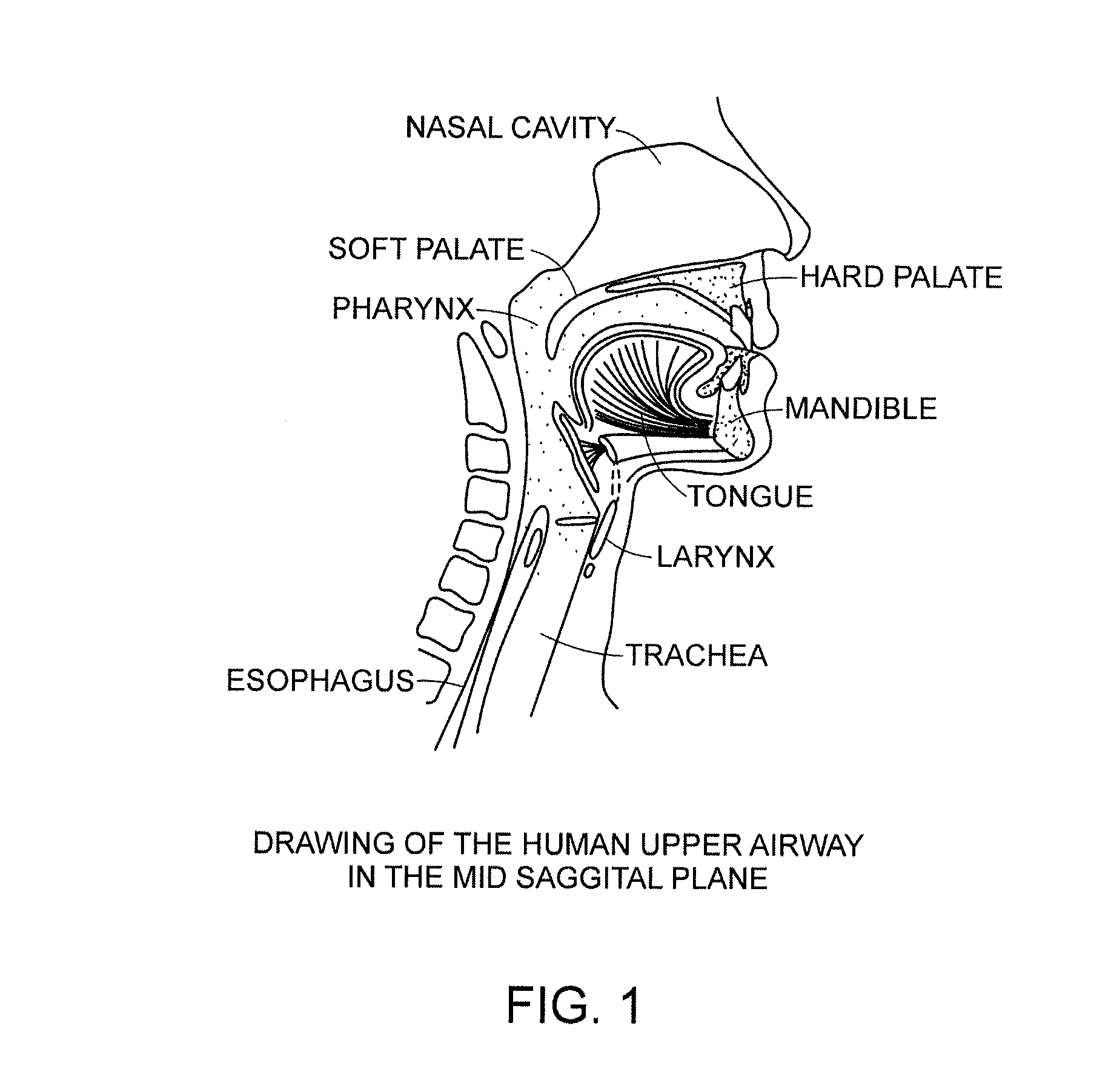
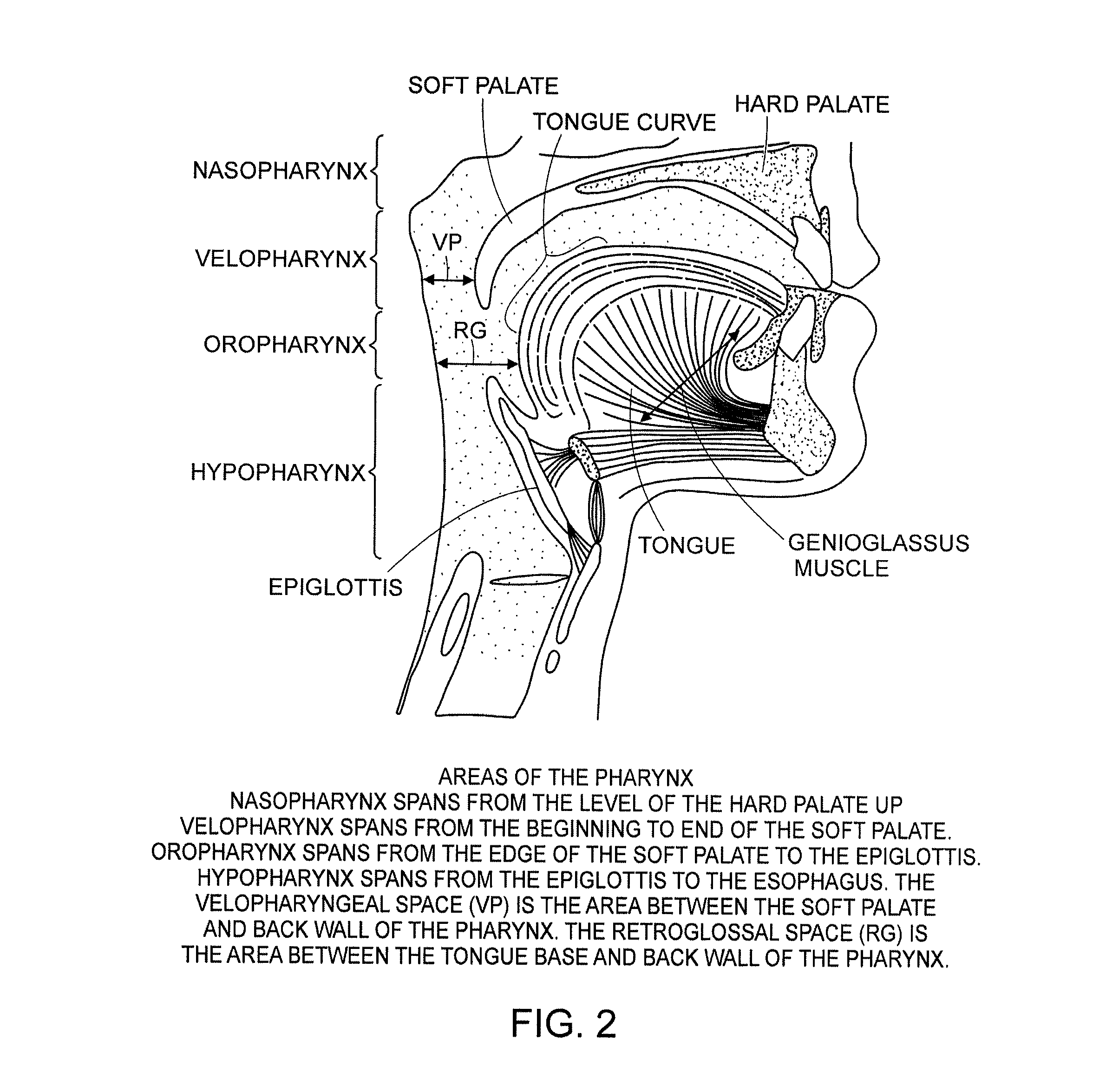
Methods and devices for treating sleep apnea and snoring
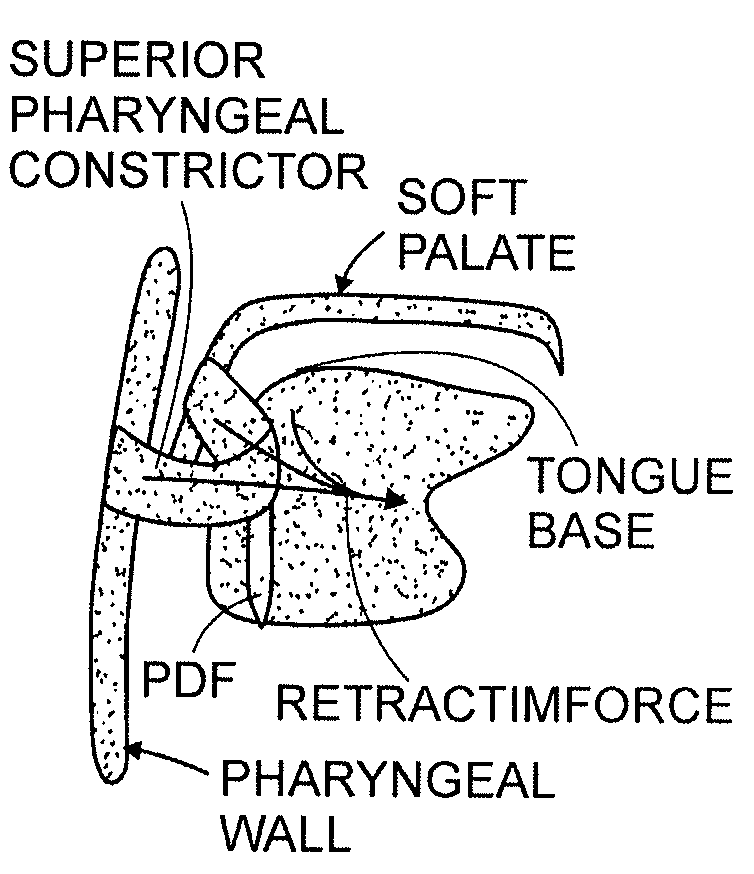
ActiveUS20080188947A1Easy to transformForce is smallSuture equipmentsDiagnosticsObstetricsBreathing disorders
Methods and devices to prevent and / or treat breathing disorders (e.g., upper airways disorders) in mammals related to impaired airflow are described. Methods and devices apply force to soft tissue that avoids obstruction of airflow in the mammel's airway. Breathing disorders that are avoided by the methods and / or devices include apnea.
Owner:LINGUAFLEX INC
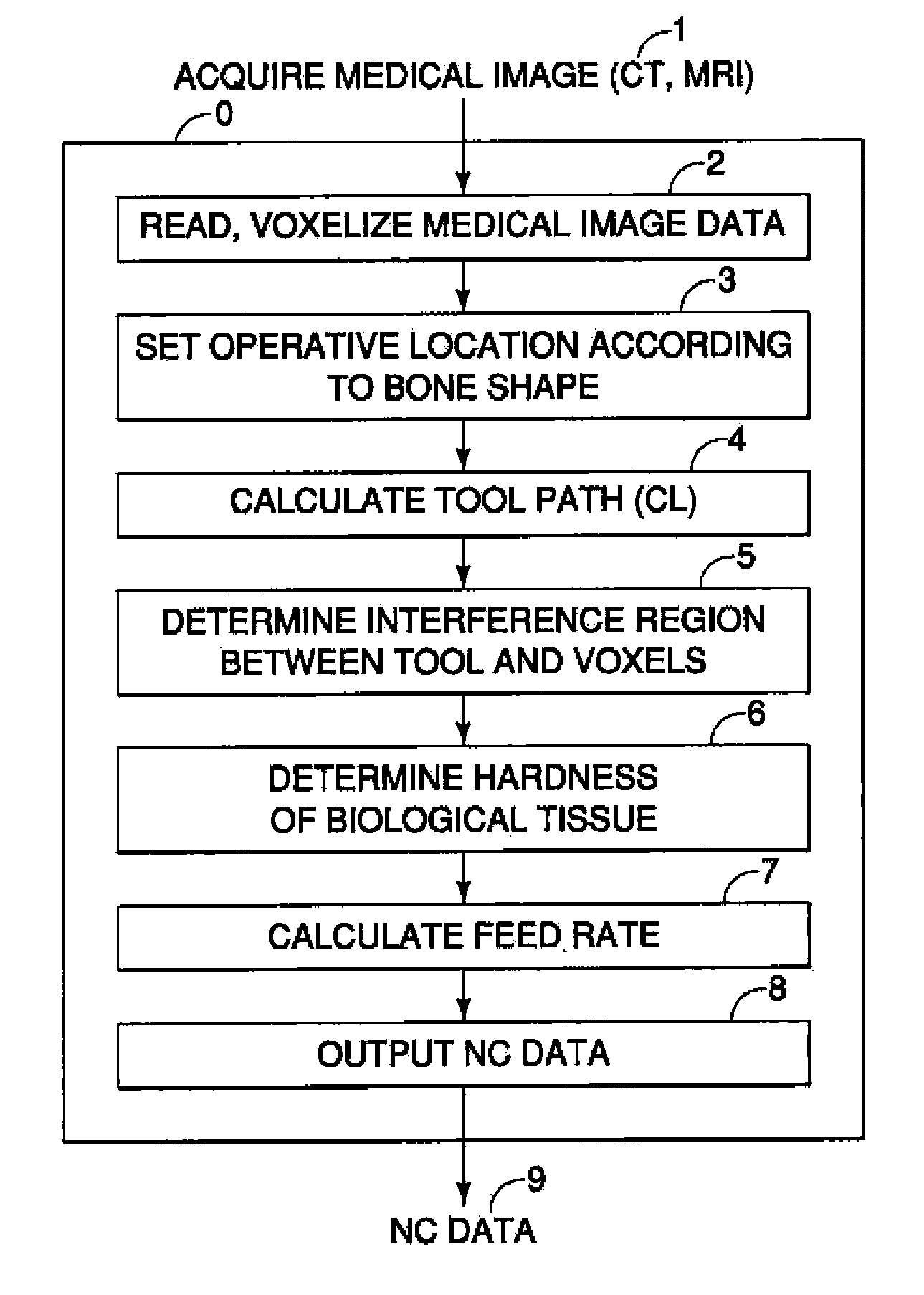
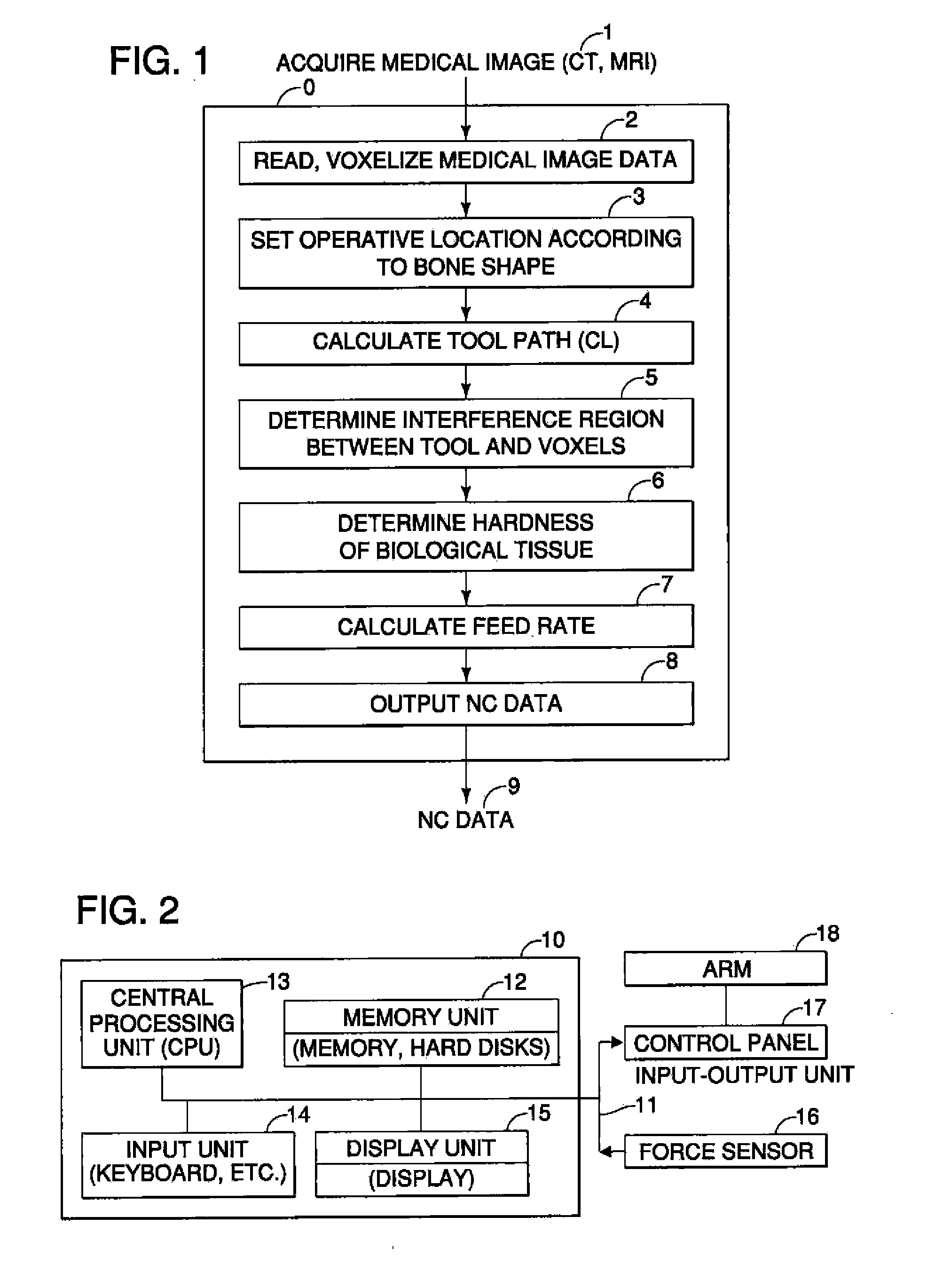
Surgical Assistance System
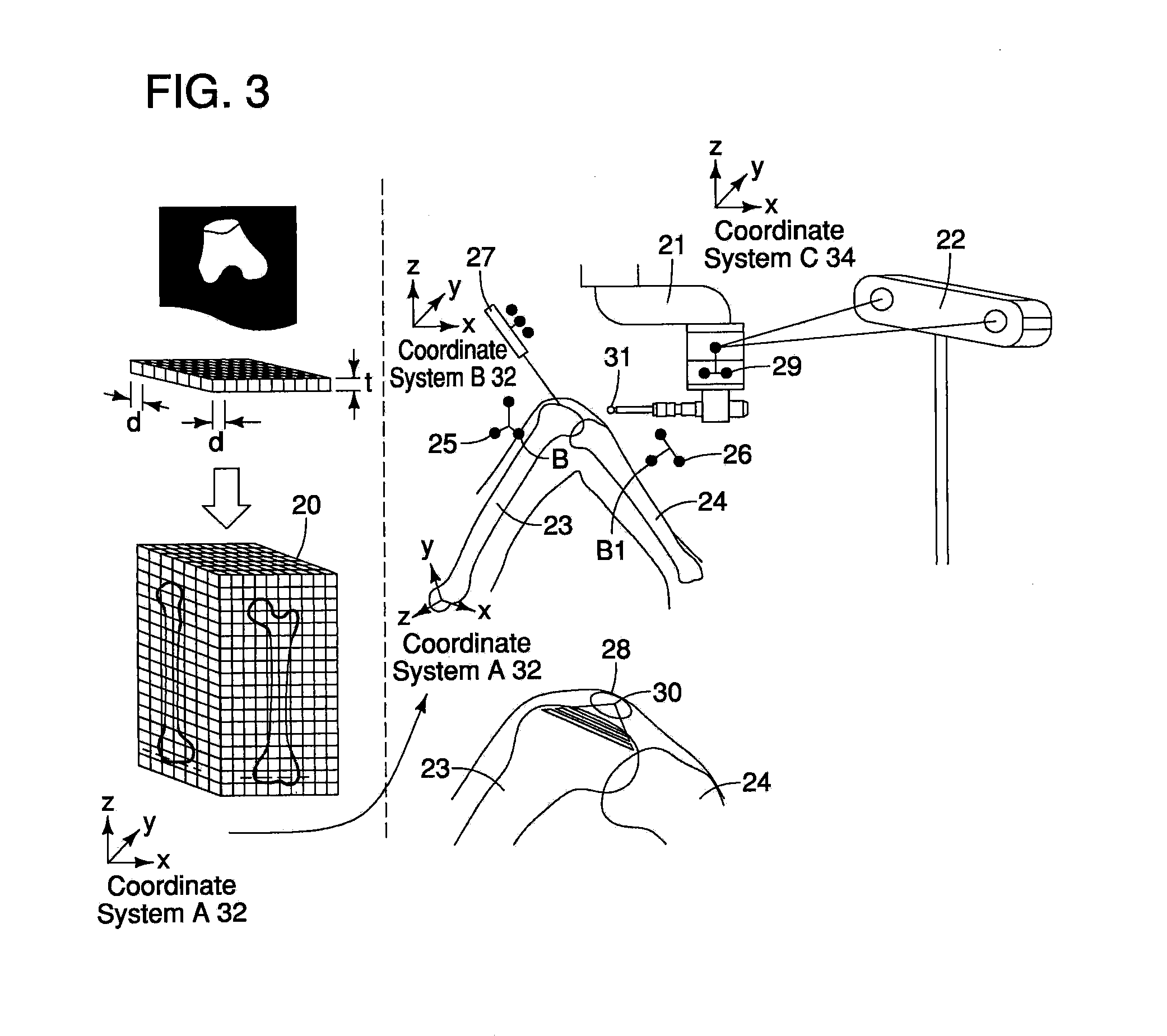
InactiveUS20110306985A1Precise resectionMinimal invasivenessDiagnosticsSurgical navigation systemsSurgical operationVoxel
A surgical assistance system for operating on biological tissue using a surgical tool attached to an arm of an automatically-controlled surgical instrument so that an optimal feed rate of the tool is calculated and outputted to the surgical instrument, the system including: a device for storing and voxelizing medical image data obtained from a biological tissue subject to surgery; a device for setting an operative location based on the shape of the biological tissue; a device for calculating a tool path along which the tool travels to perform surgery at an operative location; a device for determining the region of interference between the tool and the voxels; a device for determining the hardness of the biological tissue in the interference region; a device for calculating an optimal tool feed rate corresponding to the hardness; and a device for outputting the feed rate obtained by the calculations to the surgical instrument.
Owner:NAKASHIMA MEDICAL +1
Electrochemical Analyte Detection Apparatus and Method
InactiveUS20090026075A1Easy to useLow costImmobilised enzymesBioreactor/fermenter combinationsRedox enzymesAnalyte
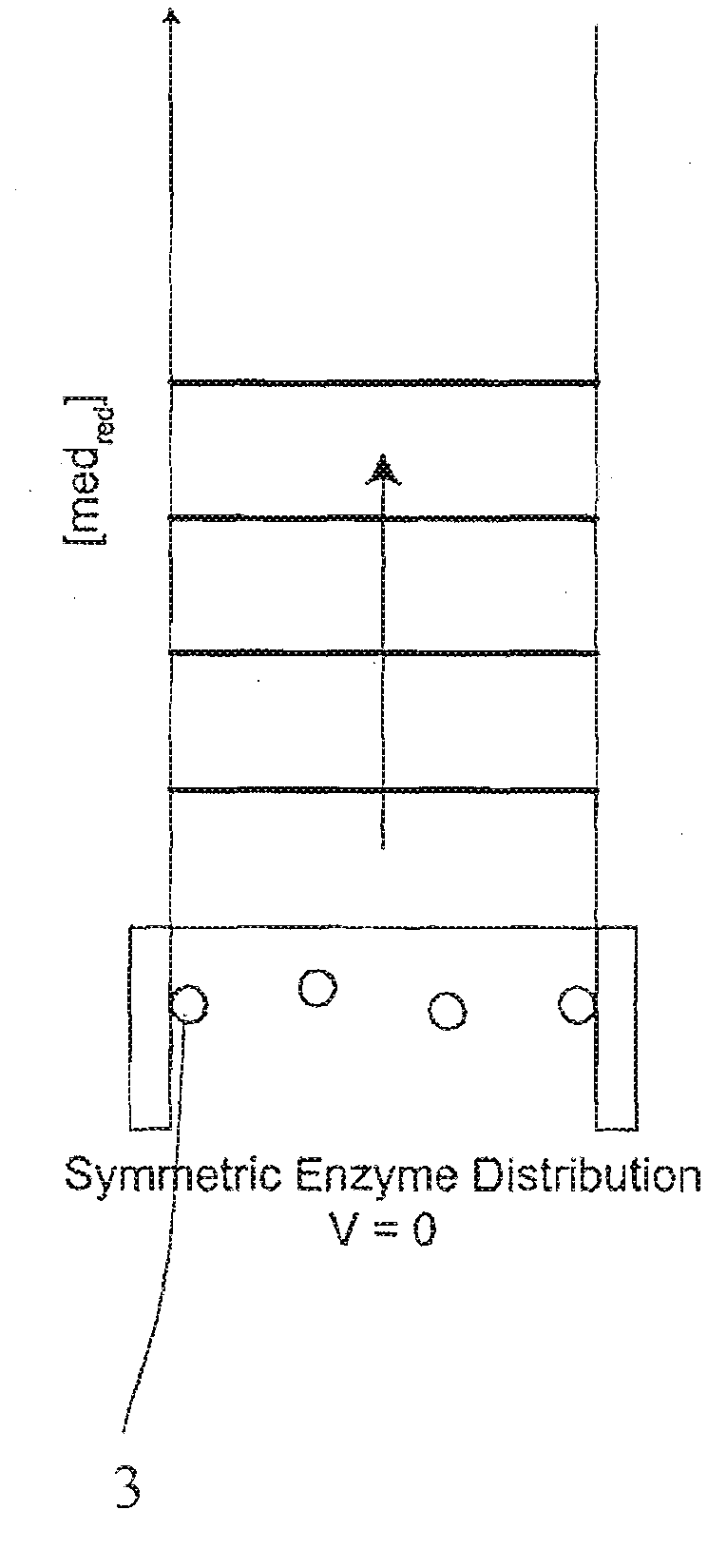
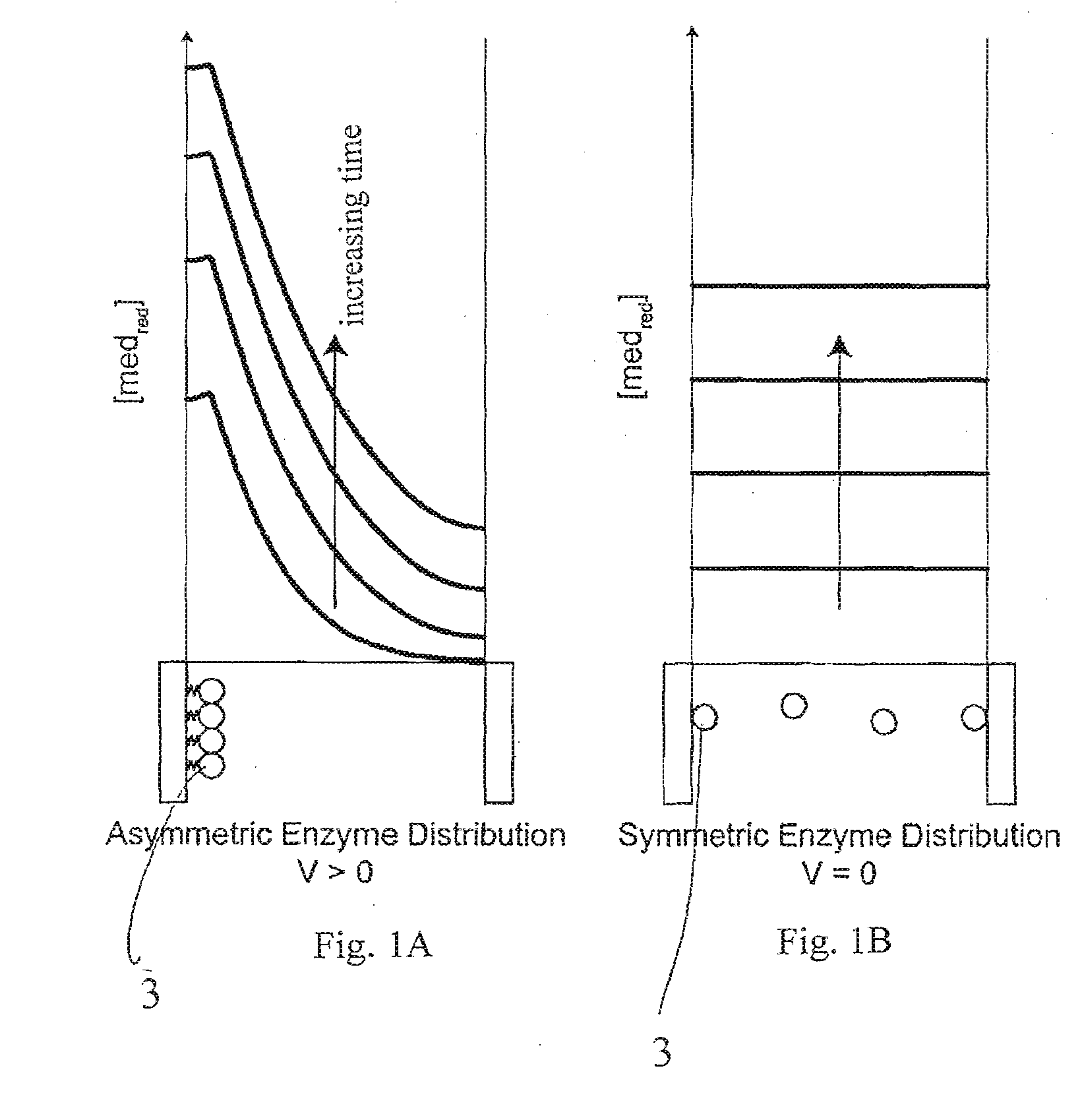
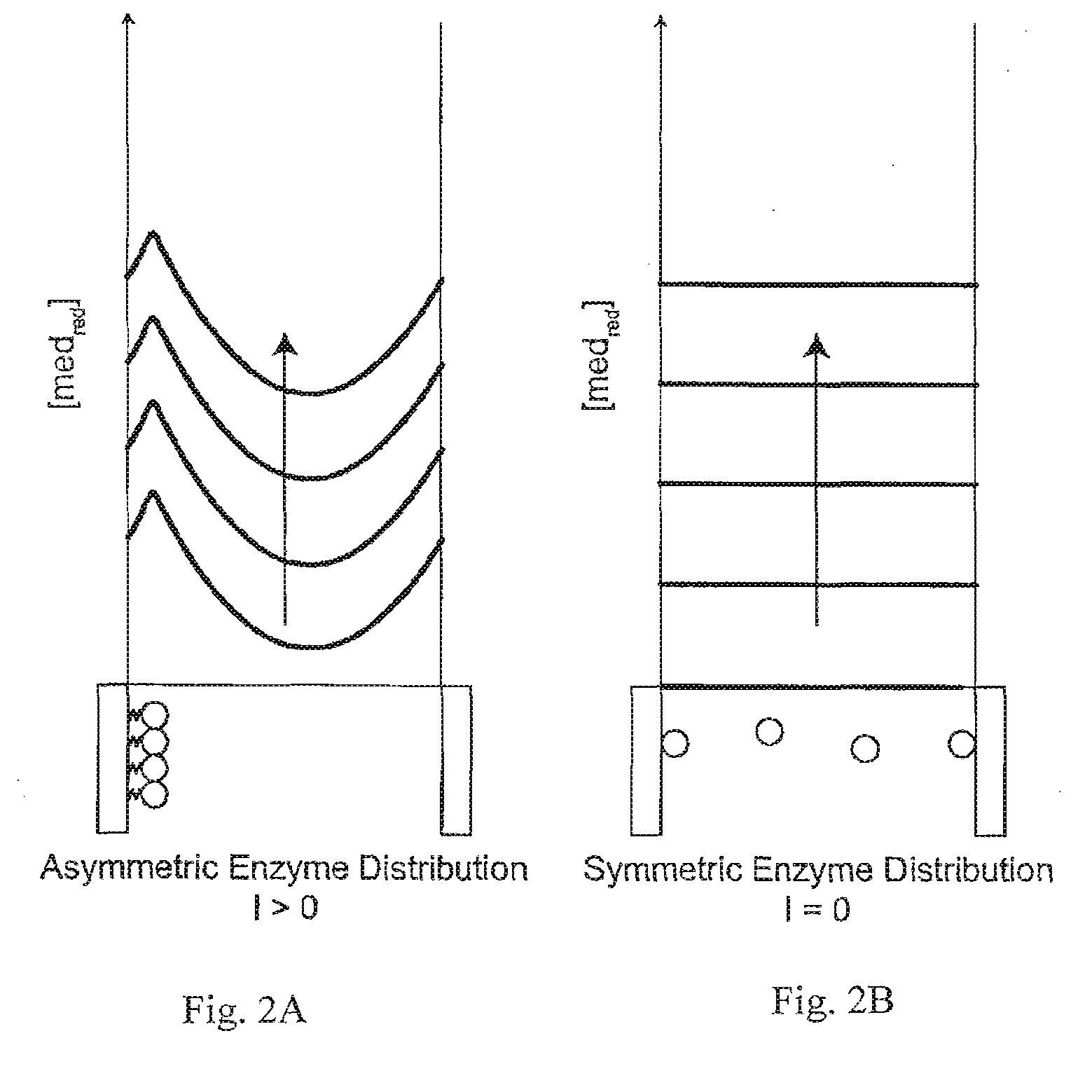
A method and apparatus for electrochemical detection of analyte in a sample makes use of a binding interaction and relies on the discovery that asymmetric distribution of a redox enzyme between two electrodes that occurs when a redox enzyme-containing reagent is immobilized at the surface of one electrode can be detected as a chemical potential gradient arising from an asymmetry, in the distribution of oxidized or reduced redox substrate. This chemical potential gradient can be detected potentiometrically by observing the potential difference between the electrodes in an open circuit, or amperometrically by observing the current flow between the electrodes when the circuit is closed. In both cases, the observation of asymmetry can be done without the application of an external potential or current to the electrodes.
Owner:AGAMATRIX INC
Fiber-optic sensing system
InactiveUS20060011820A1Low costMinimal invasivenessRadiation pyrometryFluid pressure measurement by electric/magnetic elementsFiberGrating
The invention provides a fiber-optic sensing system, utilizing a fiber-grating-based sensor, for a physical parameter, e.g., a pressure or a temperature. Different kinds of fiber-grating-based sensors may be used for this purpose but in-fiber gratings such as Fiber Bragg Grating, Long Period Grating and Surface Corrugated Long Period Fiber Grating are particularly suitable. Due to the small size of the optical fiber and the fact that same fiber acts as the sensing element as well as the signal conducting medium, it is possible to install the sensor in a small diameter needle which is commonly used for medical diagnosis and treatment. As a result, when the fiber-optic sensing system of the invention is used for in-vivo measurement of a biological parameter, such a sensing needle can be used for different in-vivo pressure or temperature sensing applications without causing too much harm and discomfort to the subject tested.
Owner:YIP KIN MAN +1
Therapeutic Delivery Device
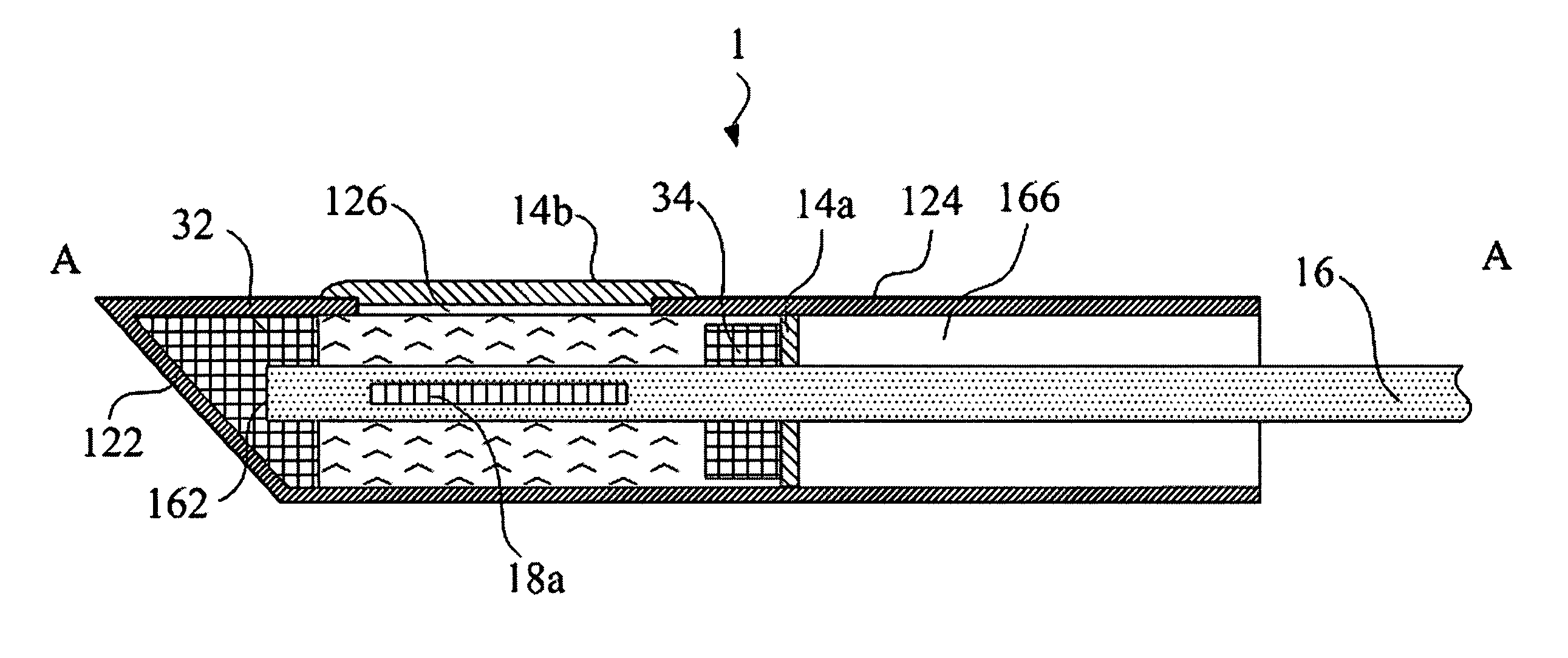
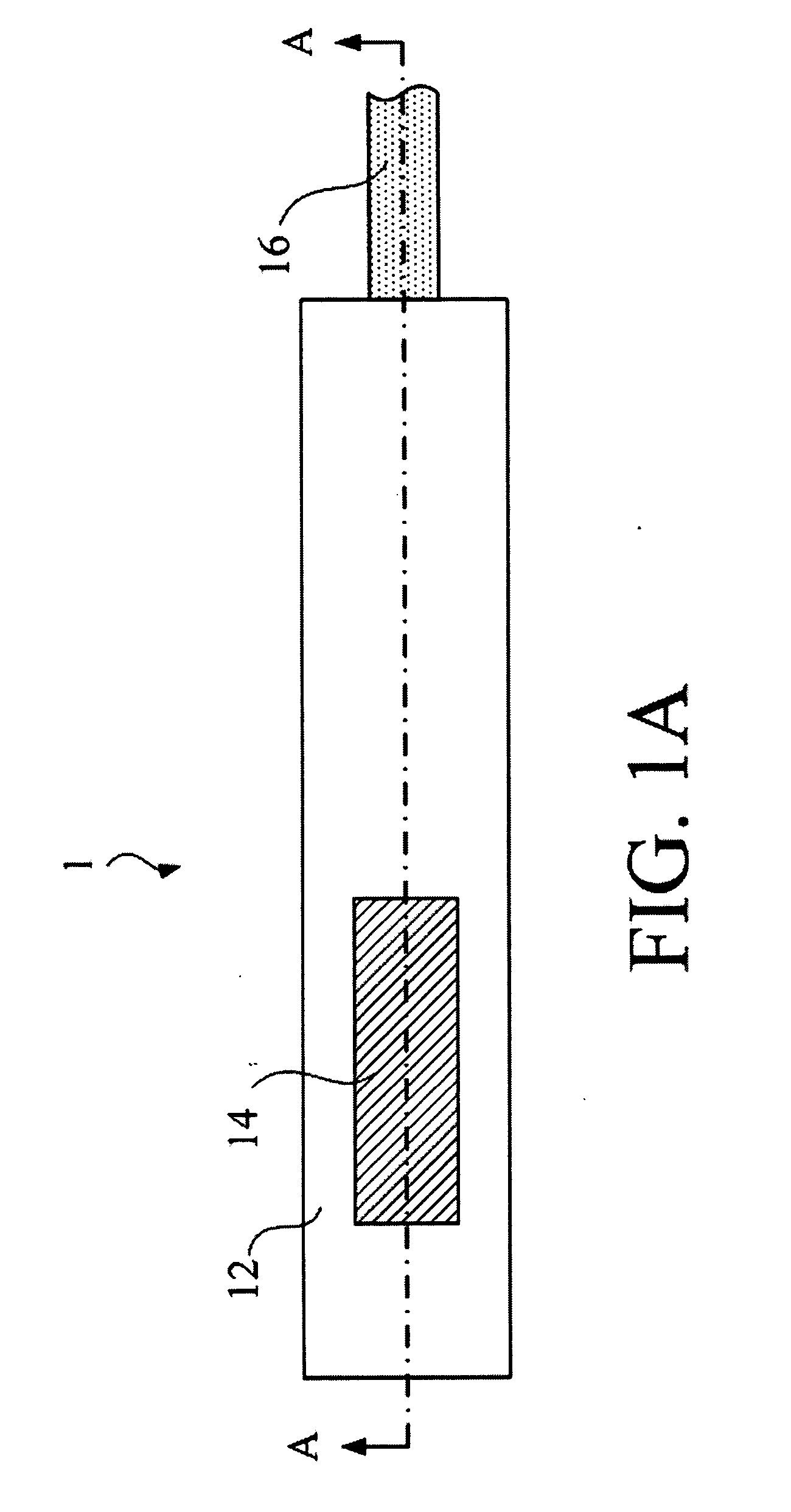
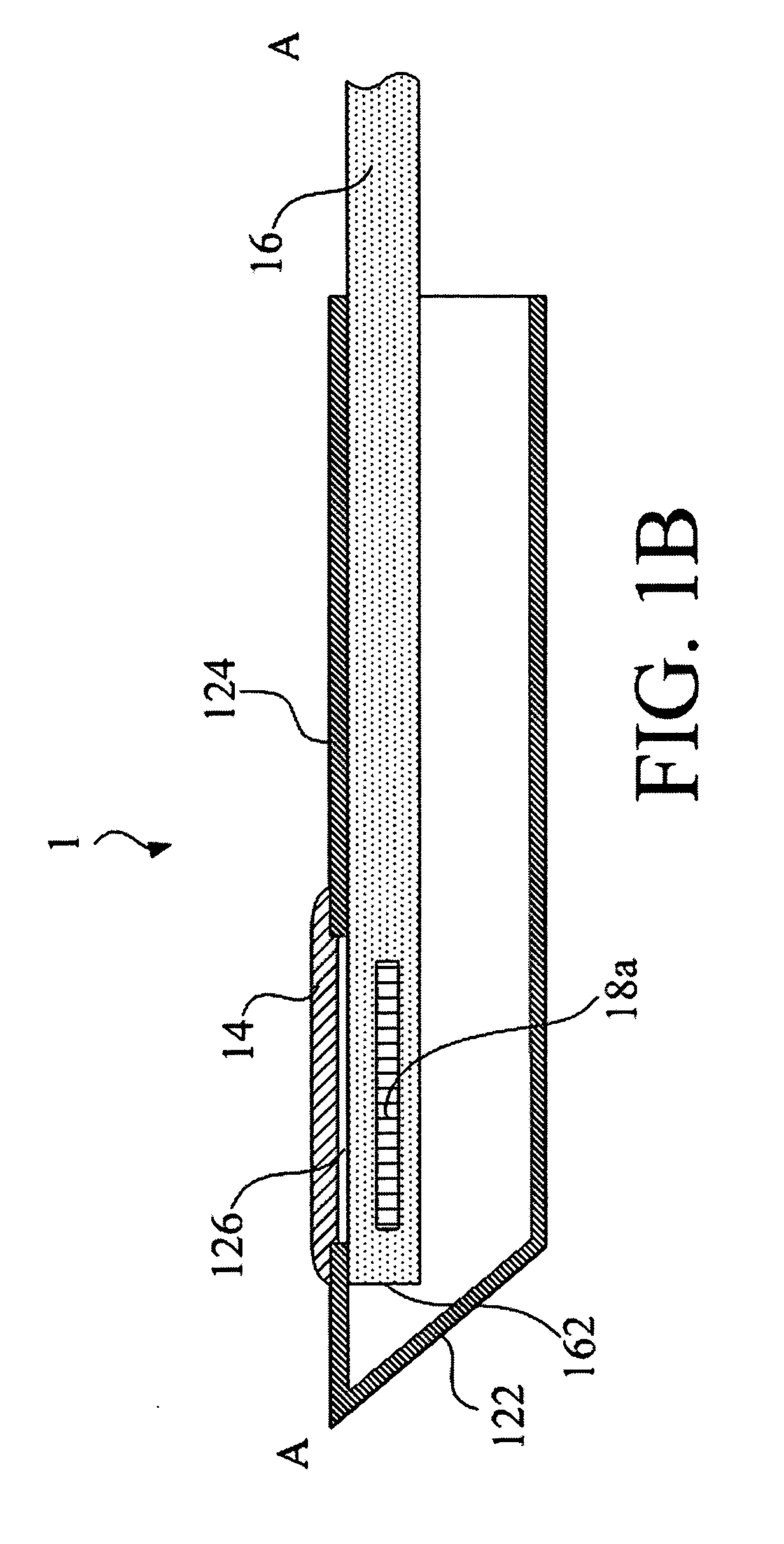
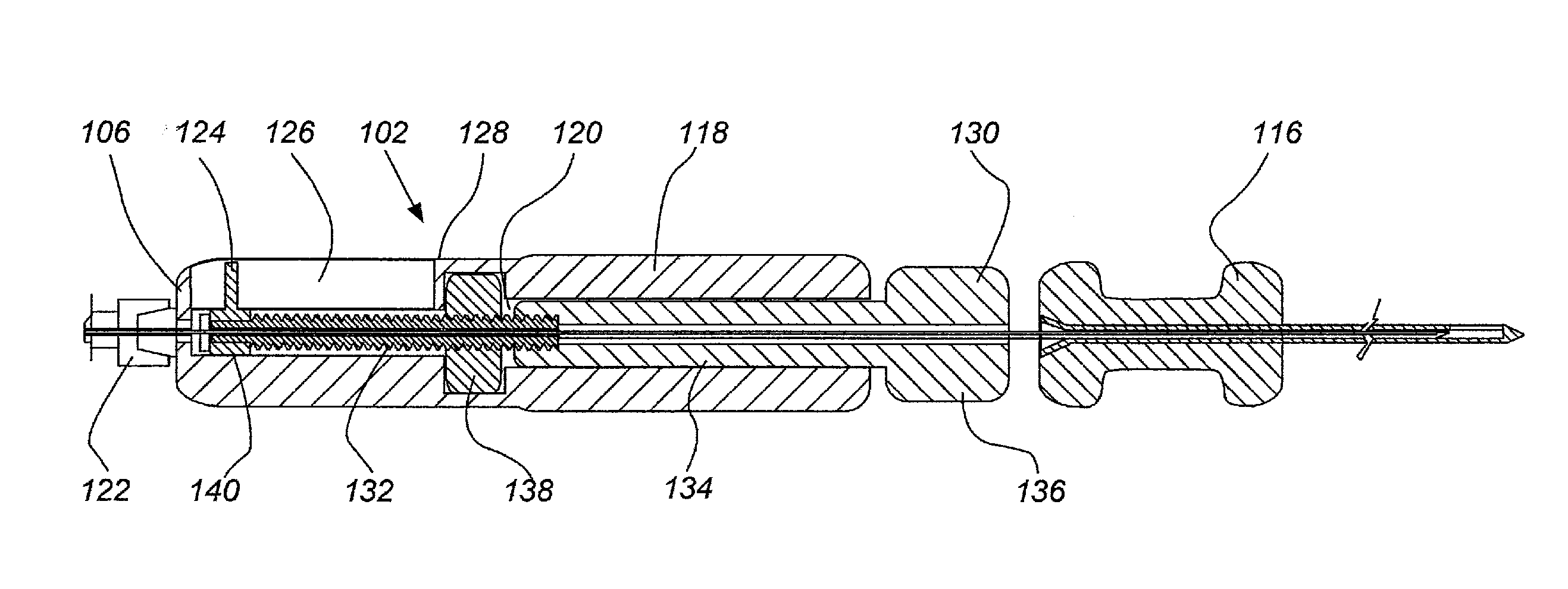
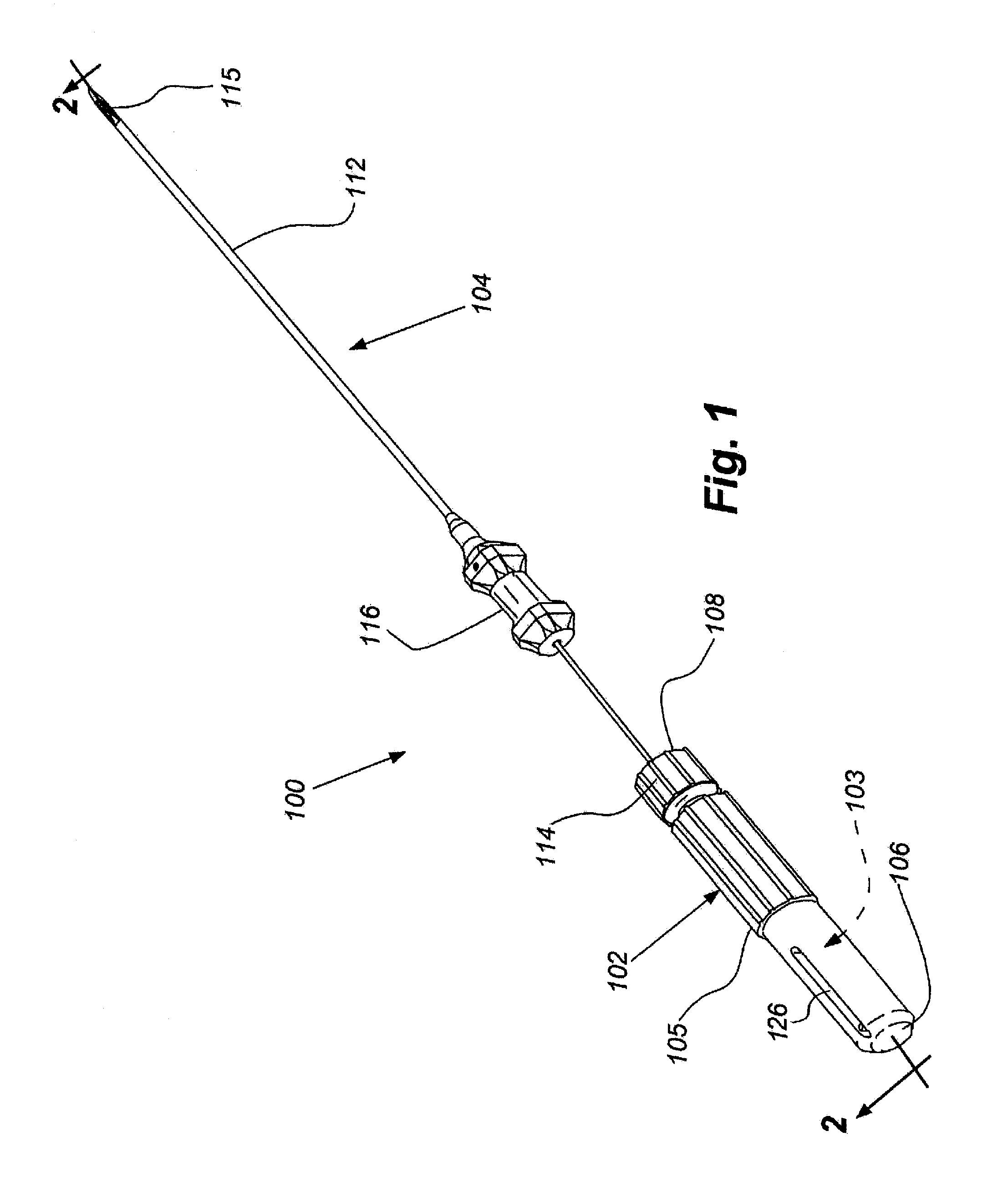
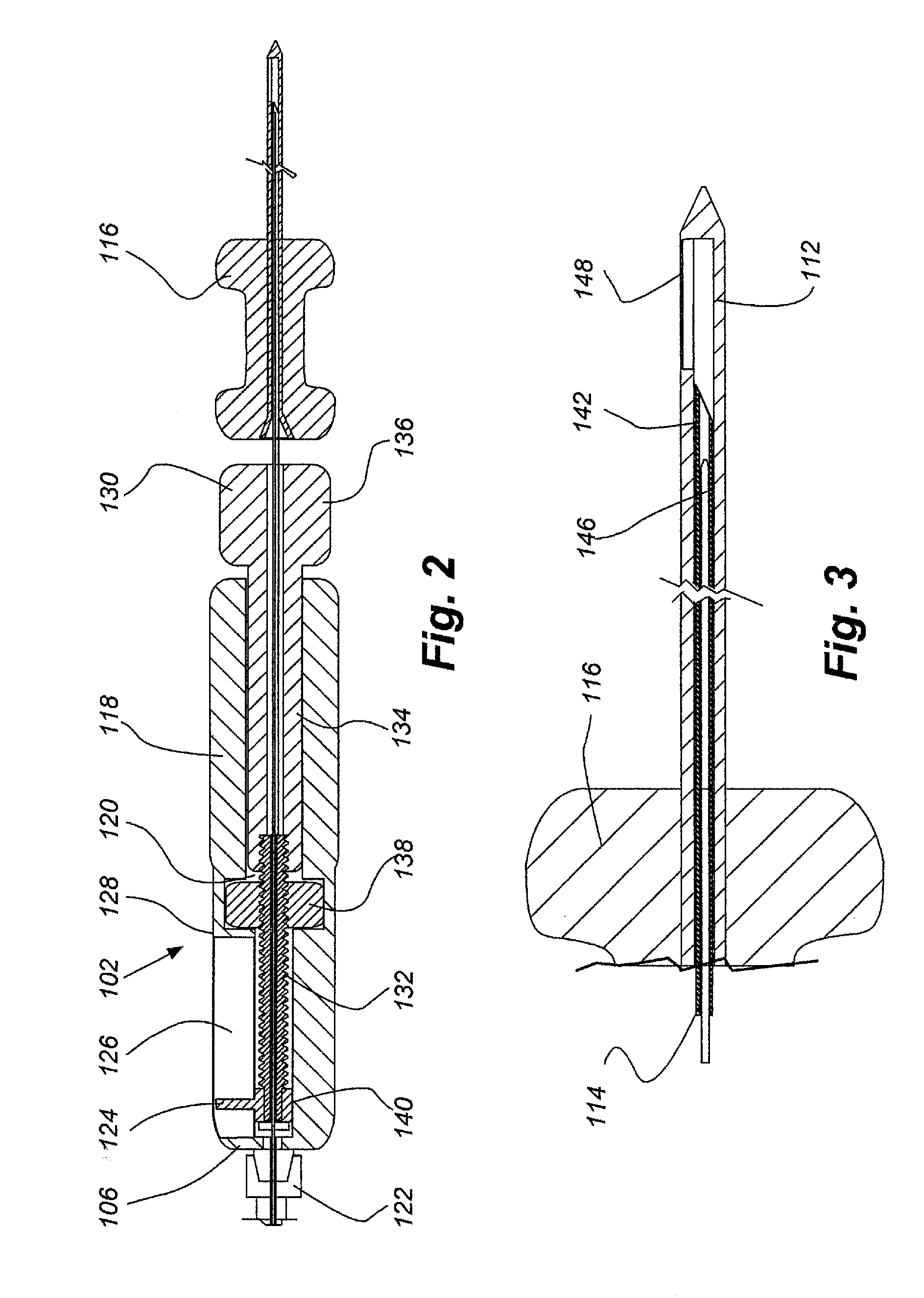
ActiveUS20110276001A1Minimal invasivenessAccurate delivery of therapeuticInfusion syringesSurgical needlesIntervertebral discBiomedical engineering
Methods and device are provided for delivery of biologics to an intervertebral disc. Device described herein include a needle assembly and handle assembly that optimize biologic delivery parameters to a target location within a disc. The needle assembly includes the capability of advancing into the disc at pre-configured pathways that allow for minimized damaged and maximized positioning of the biologic.
Owner:REGENEXX LLC
Surgical procedure for correcting cystocele and rectocele
InactiveUS20090105526A1Minimal invasivenessRapid and less painfulAnti-incontinence devicesLower poleSurgical department
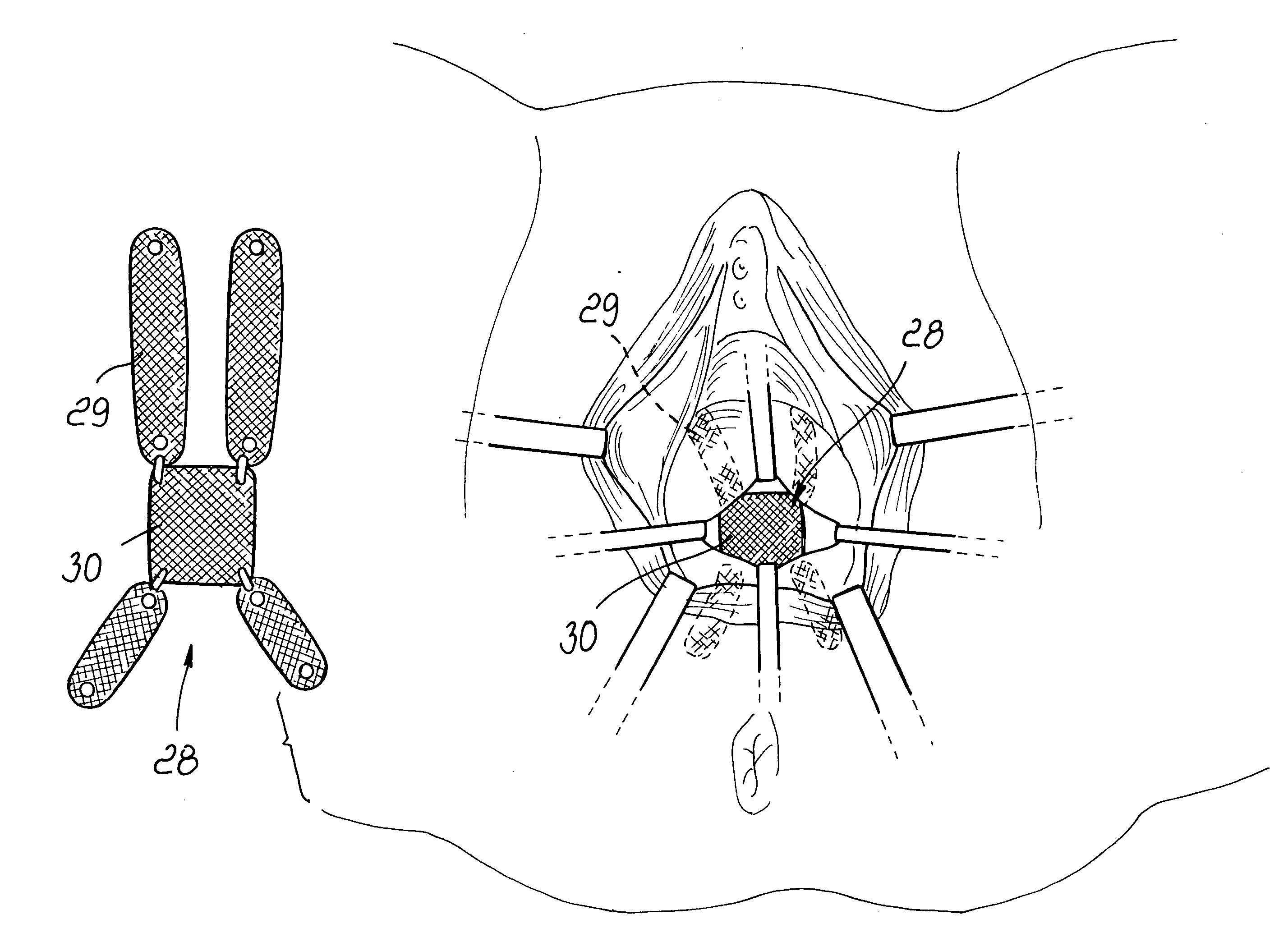
A surgical procedure for correcting cystocele, comprising the steps of:identifying a center of the cystocele;performing an infiltration of the cystocele with physiological solution;performing a longitudinal incision;identifying the pubocervical fascia and performing a longitudinal incision therein;defining four paravesical tunnels directed toward the upper and lower poles of the obturator foramina bilaterally;introducing a mesh with four articulated arms, each arm being introduced in a corresponding tunnel;suturing the longitudinal incision.
Owner:PIROLI TORELLI DONATO +1
Implantable systems and stents containing cells for therapeutic uses
InactiveUS20060136049A1Improve personal protectionLess invasiveStentsFiltering accessoriesInsertion stentImplanted device
An implantable system includes cells that produce and release a therapeutic agent, or agents, to a host in need. The system can include cells capable of eluting therapeutic agents in response to changing physiological conditions within a host. The system may comprise naked cells, encapsulated cells, or a mixture of non-encapsulating and encapsulated cells. The system may also comprise cells, and / or cell groups, of different origins. The implantable device may be placed intra-vascular, within bone marrow, within soft tissue, in the peritoneal cavity, or intra-hepatic, etc. The system can be comprised of a stent, or like devices. Such stents and like devices may optionally include port(s), catheter(s), and containment envelope systems for holding the above cells.
Owner:ROJO NICHOLAS A
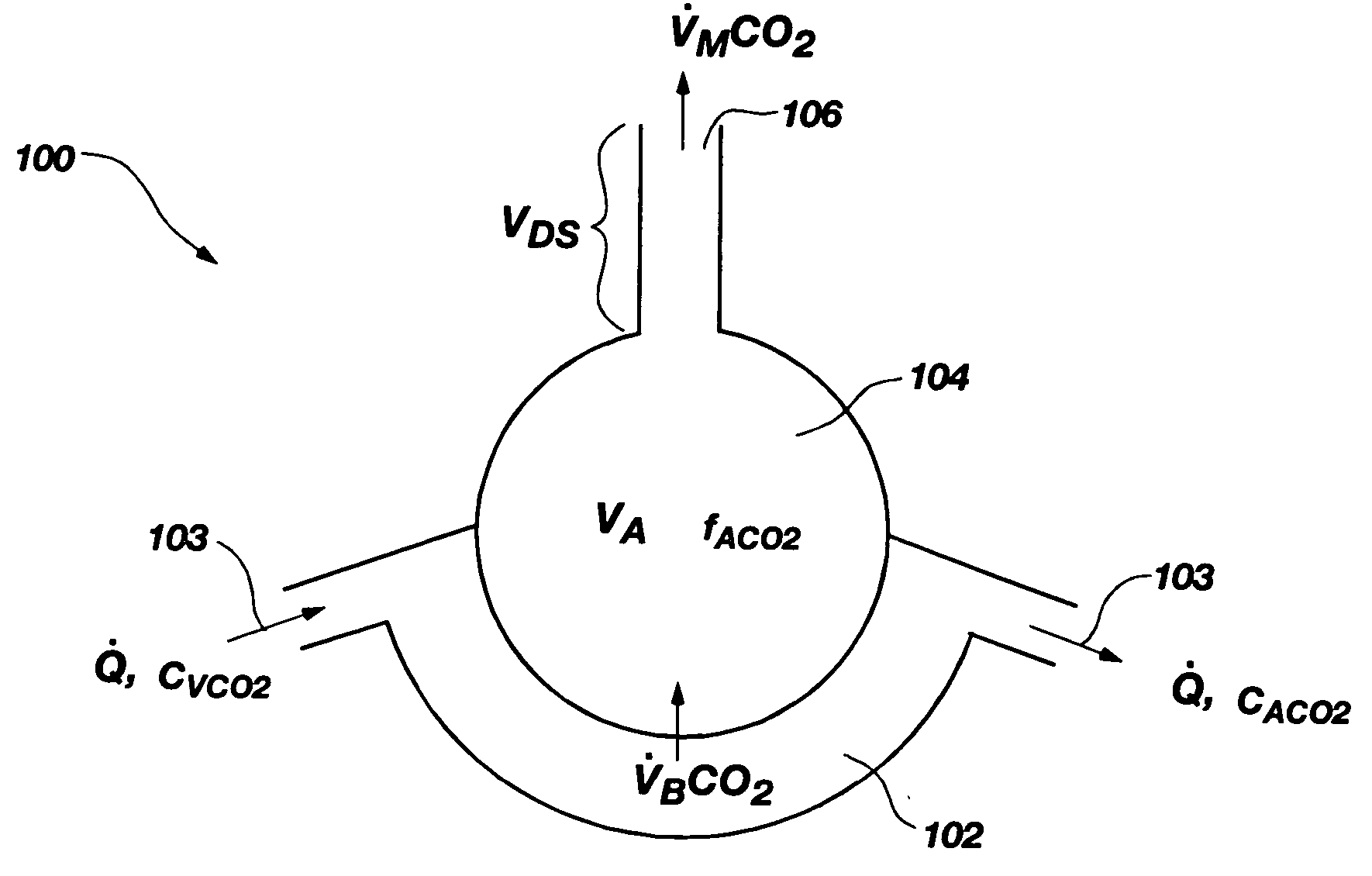
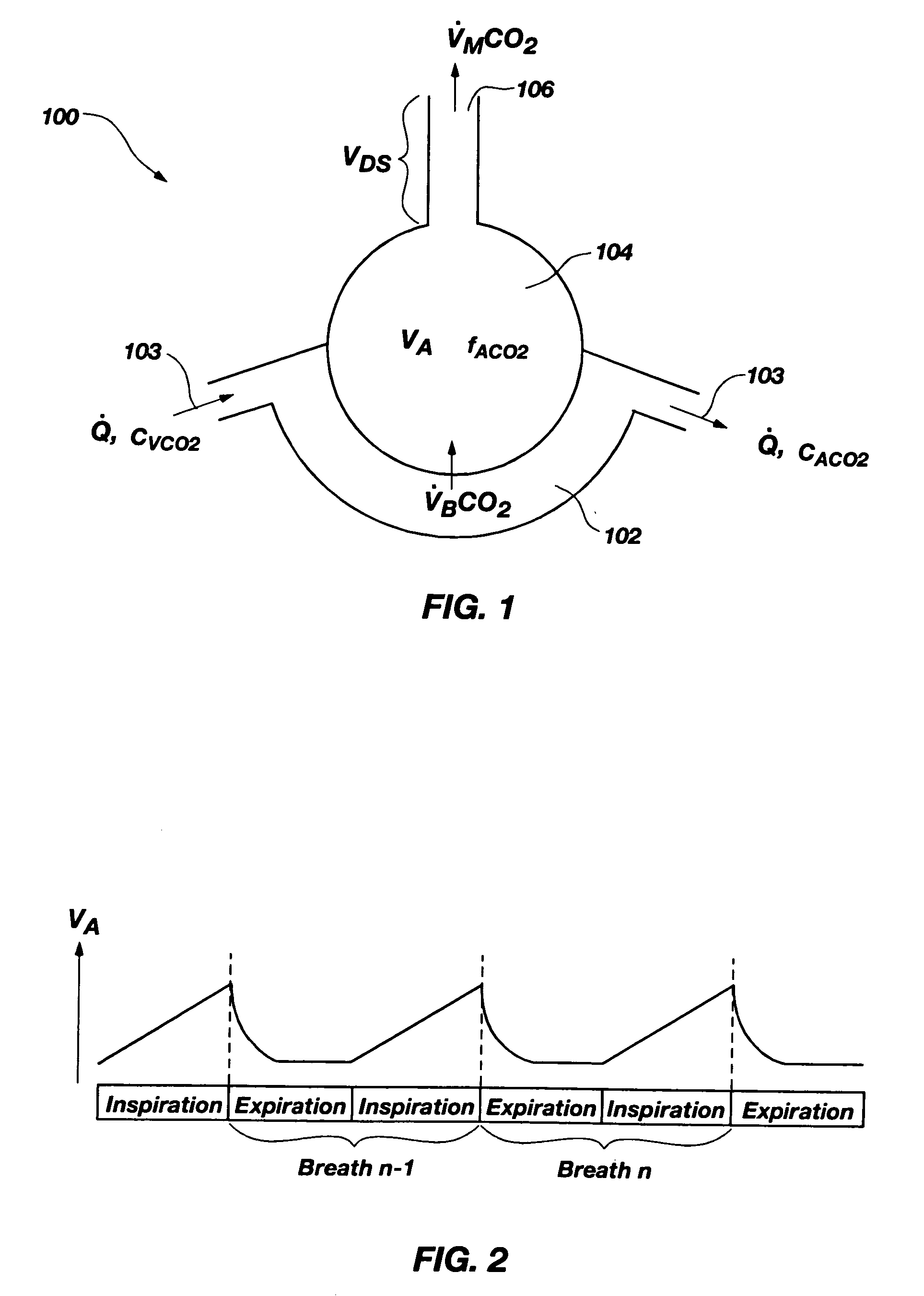
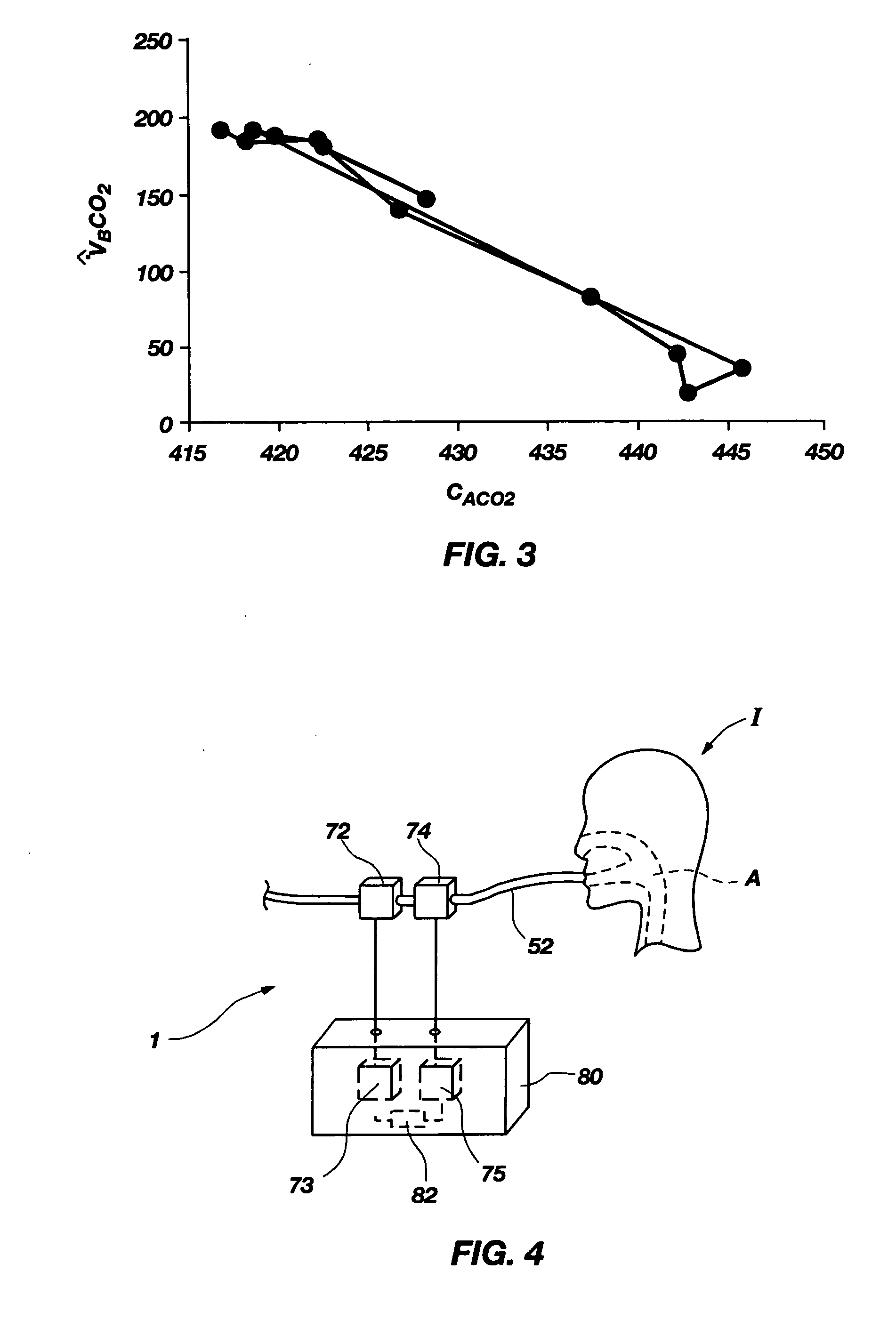
Noninvasive effective lung volume estimation
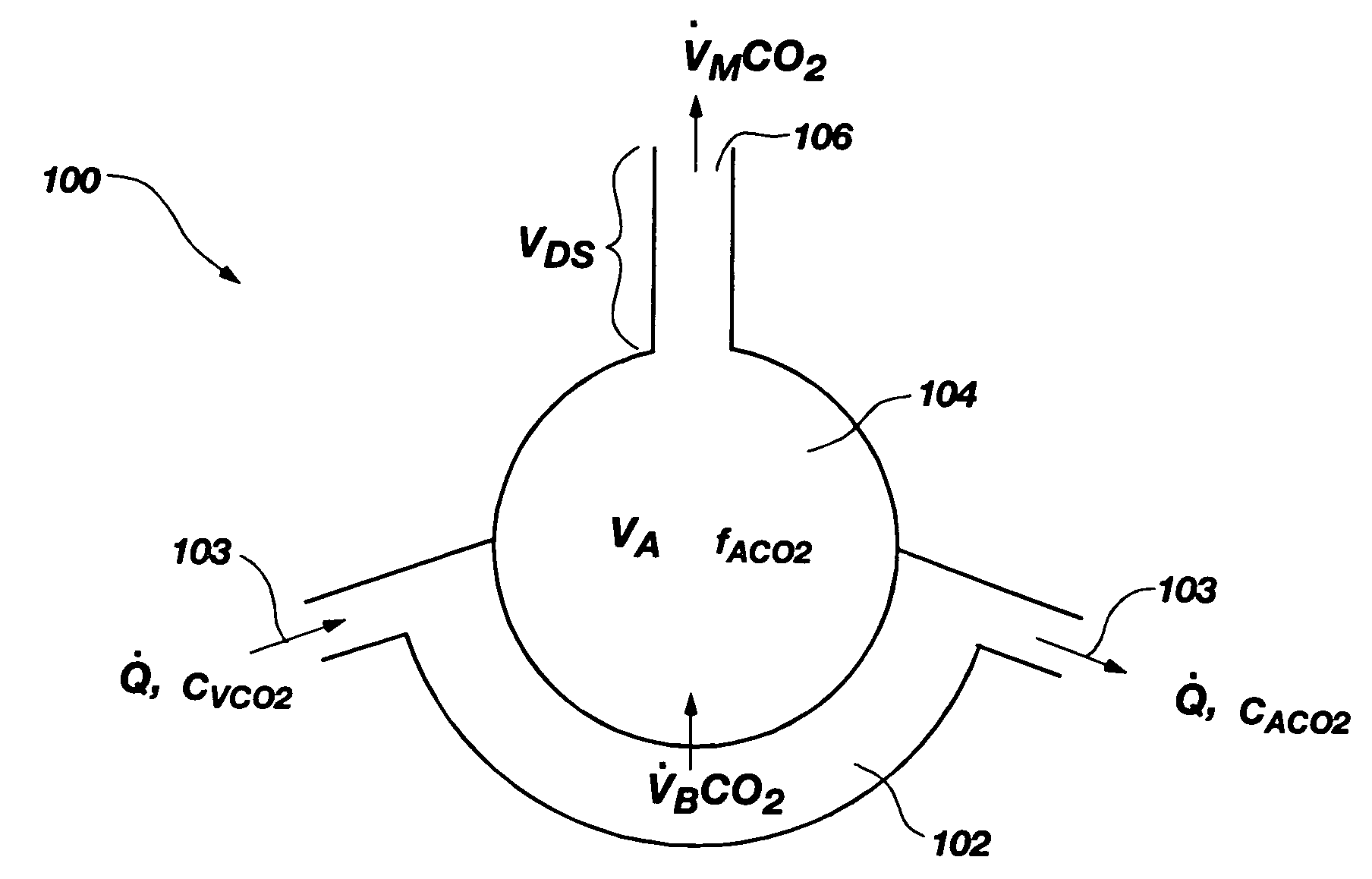
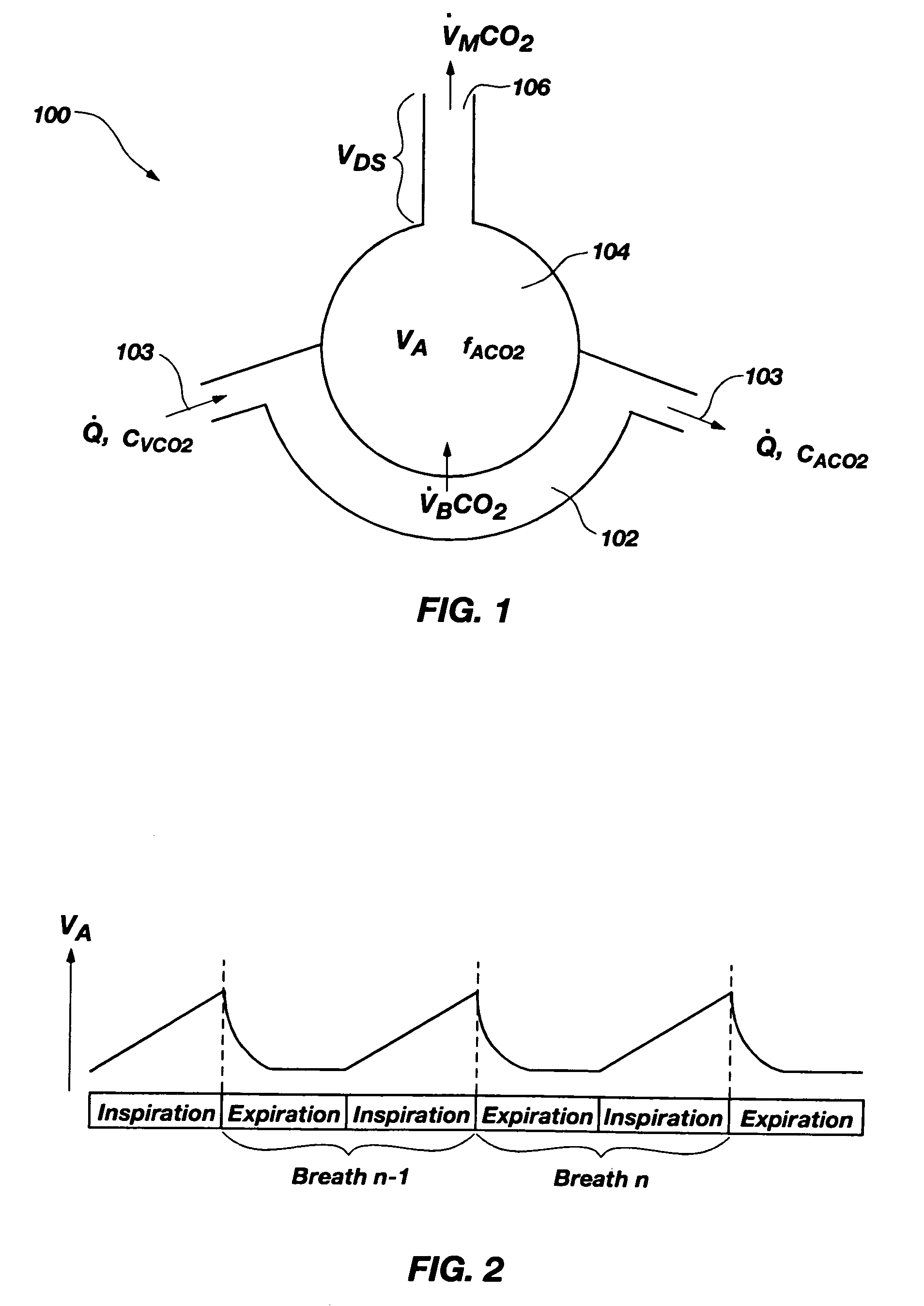
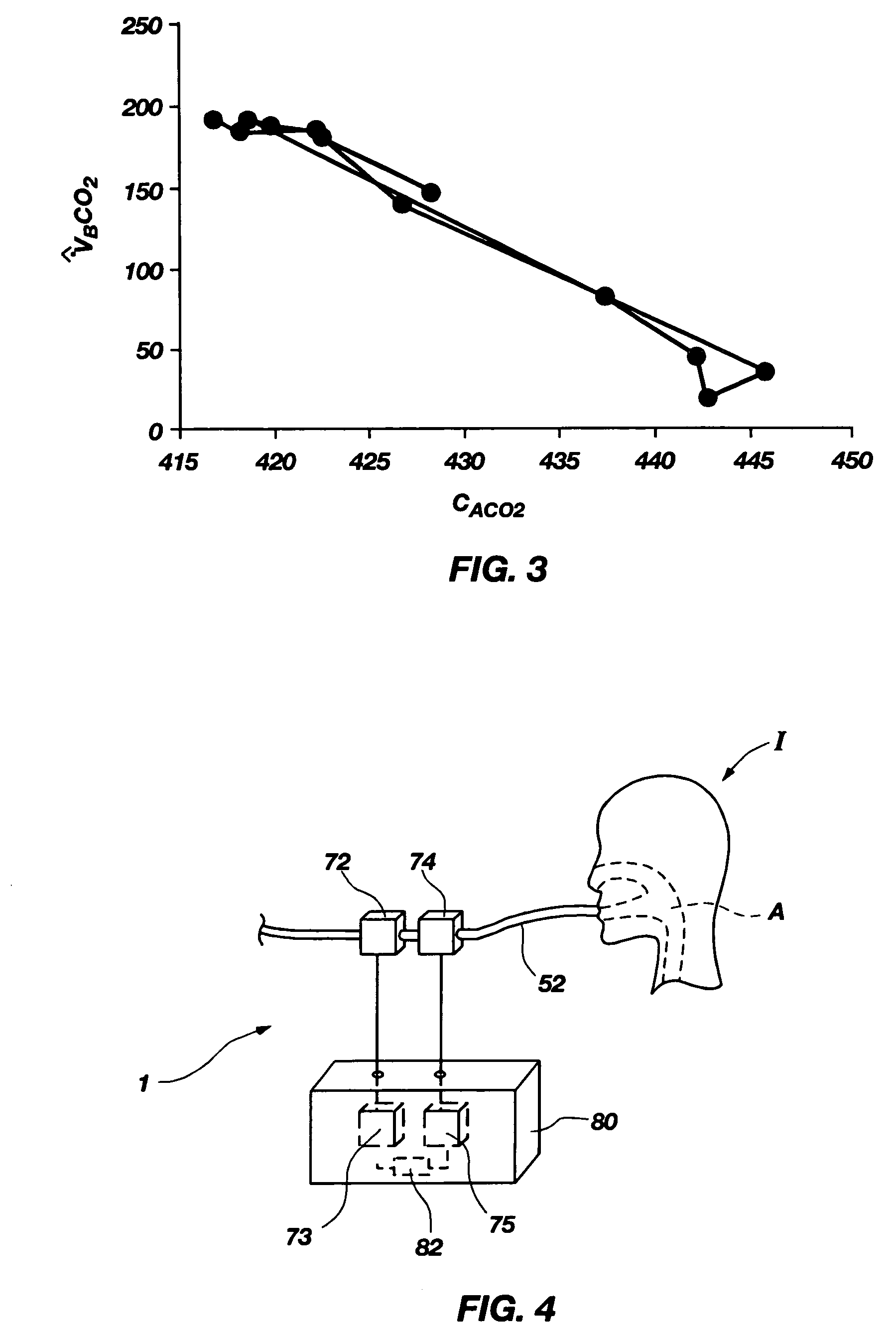
InactiveUS7699788B2Minimal invasivenessWithdrawing sample devicesRespiratory organ evaluationEstimation methodsIntensive care medicine
Methods for noninvasively measuring, or estimating, functional residual capacity or effective lung volume include obtaining carbon dioxide and flow measurements at or near the mouth of a subject. Such measurements are obtained during baseline breathing and during and shortly after inducement of a change in the subject's effective ventilation. The obtained measurements are evaluated to determine the amount of time required for exhaled carbon dioxide levels to return to normal—effectively an evaluation of carbon dioxide “washout” from the subject's lungs. Conversely, carbon dioxide and flow measurements may be evaluated to determine the amount of time it takes carbon dioxide to “wash in,” or reach peak levels within, the lungs of the subject following the change in the subject's effective ventilation. Apparatus for effective such methods are also disclosed.
Owner:RIC INVESTMENTS LLC
Methods and devices for treating sleep apnea and snoring
ActiveUS8074655B2Easy to transformForce is smallSuture equipmentsDiagnosticsObstetricsBreathing disorders
Methods and devices to prevent and / or treat breathing disorders (e.g., upper airways disorders) in mammals related to impaired airflow are described. Methods and devices apply force to soft tissue that avoids obstruction of airflow in the mammel's airway. Breathing disorders that are avoided by the methods and / or devices include apnea.
Owner:LINGUAFLEX INC
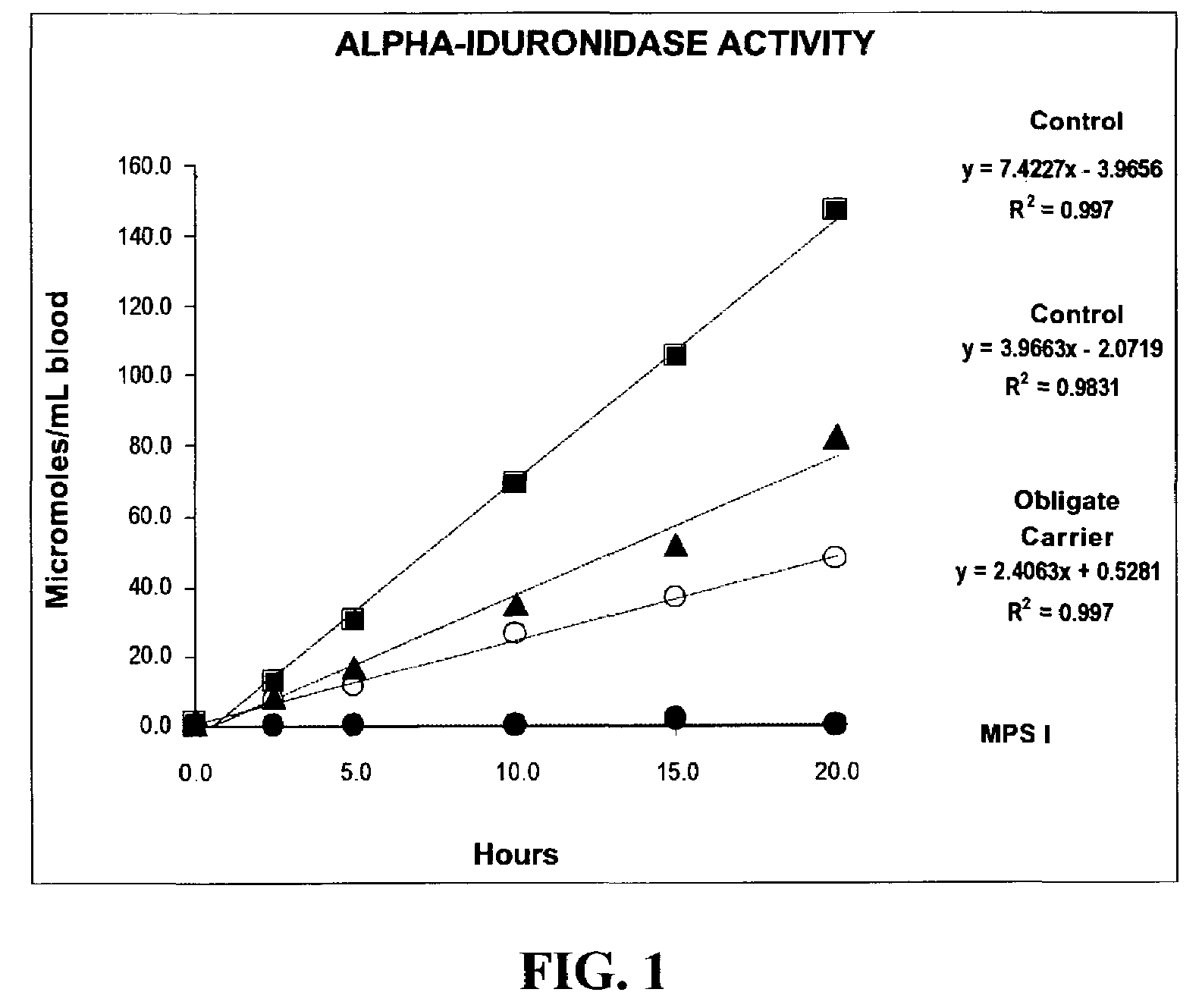
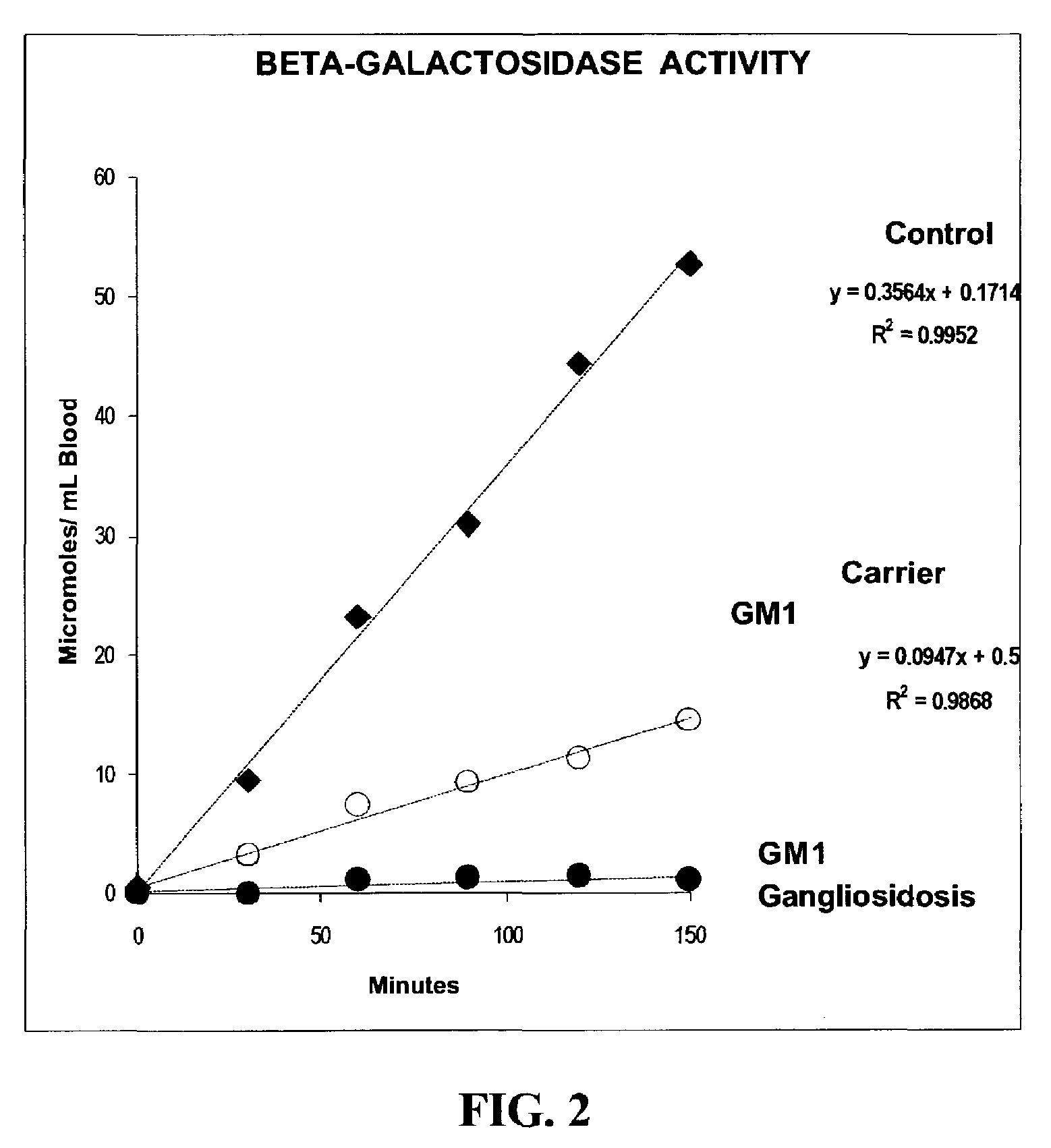
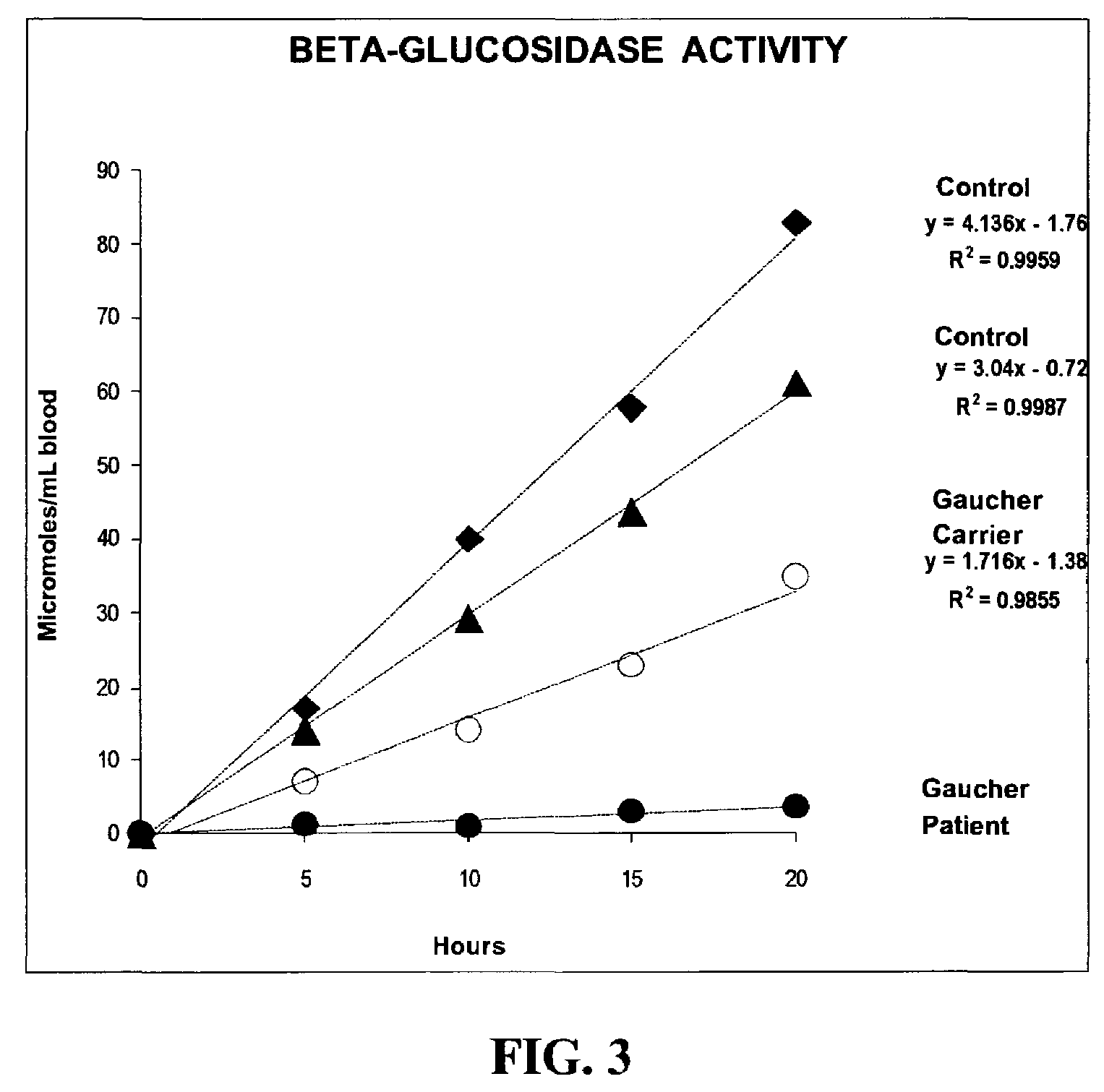
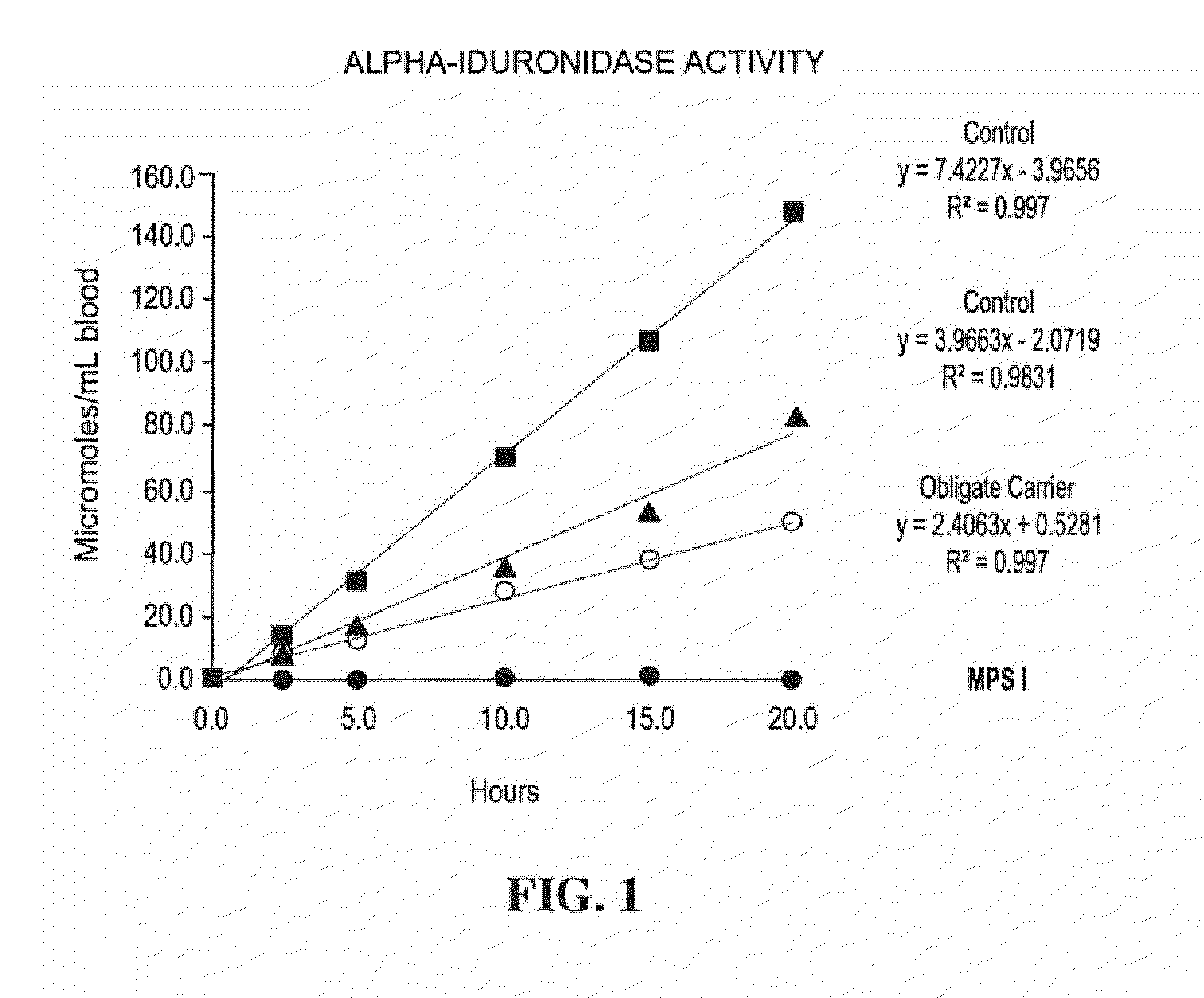
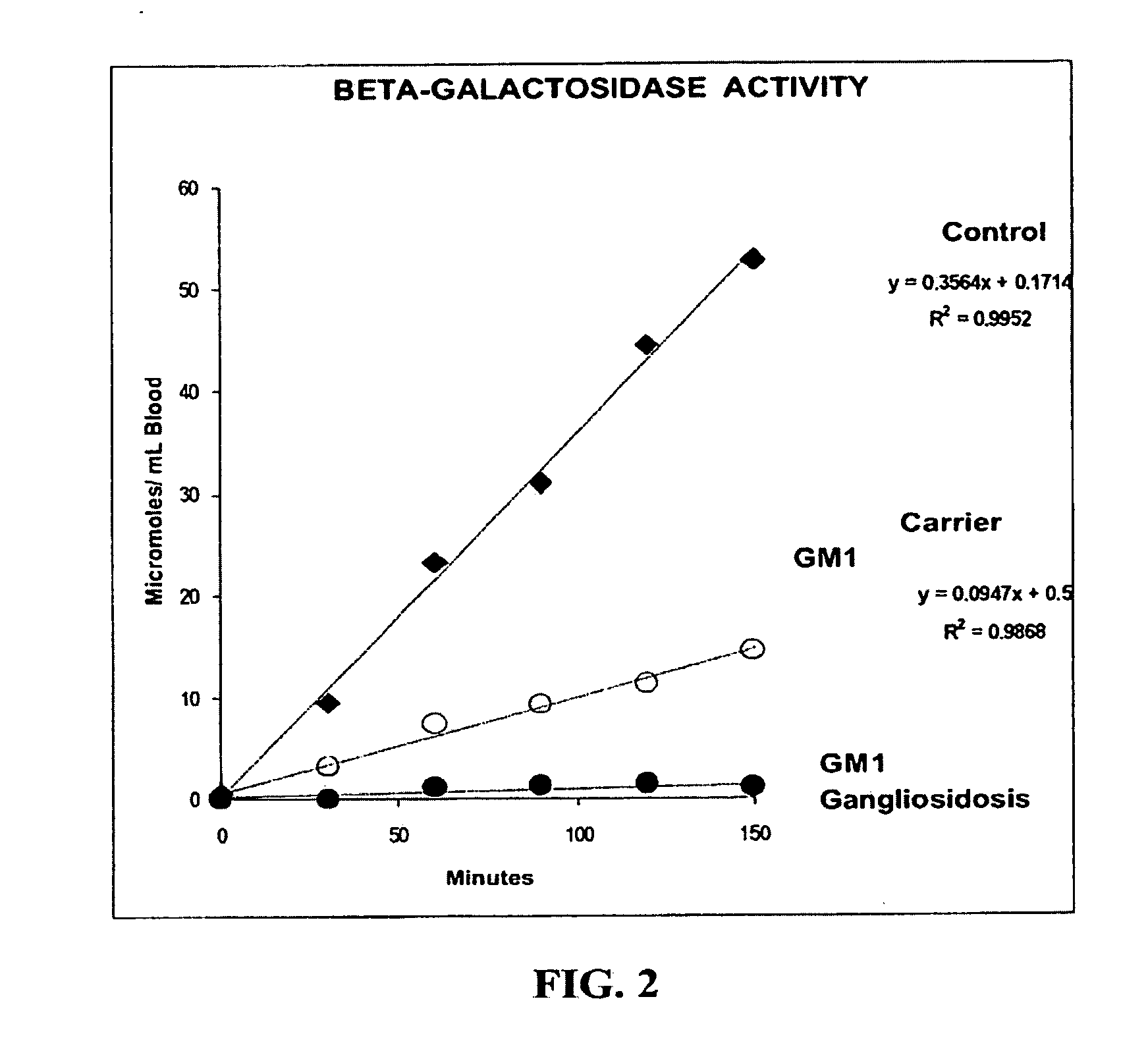
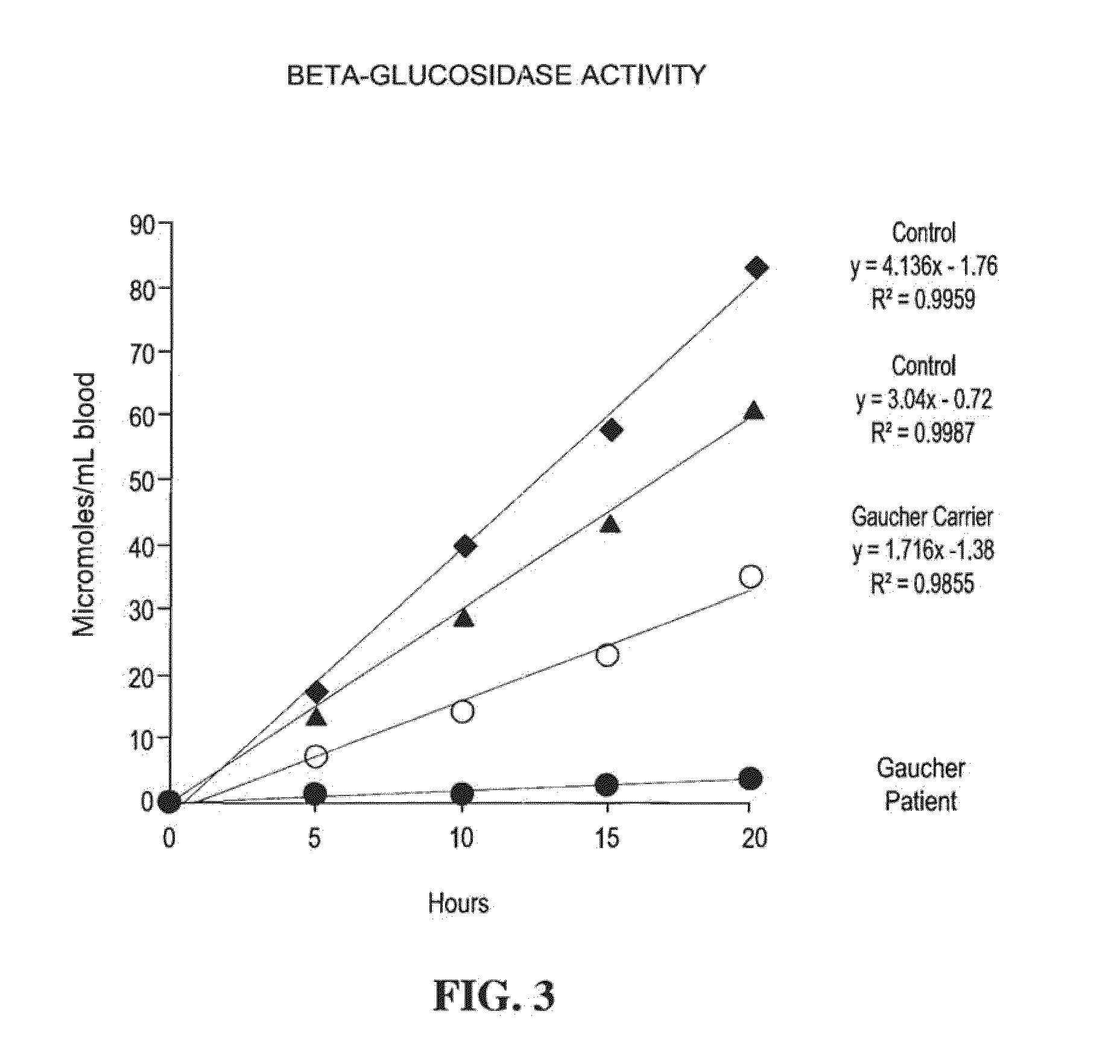
Method for assaying the activity of lysosomal enzymes
InactiveUS7563591B2Inexpensive and simple for determinationSimple and expedite sample collectionMicrobiological testing/measurementPreparing sample for investigationIduronidaseSufficient time
A method, and associated kit, for assaying the activity of lysosomal enzymes present in dried bodily fluids and cell tissue samples, such as α-L-iduronidase, β-D-galactosidase, β-D-glucosidase, chitotriosidase, total α-D-galactosidase and α-D-galactosidase A, hexosaminidase A and B, α-D-mannosidase, β-D-mannosidase, α-L-fucosidase, N-acetyl-α-galactosaminidase, arylsulfatases, sphingomyelinase, β-galactocerebrosidase, iduronate-2-sulfatase and β-D-glucuronidase. The method includes: (a) combining with a dried bodily fluid or cell tissue sample containing at least one type of lysosomal enzyme: (1) an eluent, (2) an incubation buffer and (3) a substrate or substrates capable of reacting with the assayed lysosomal enzymes and producing their corresponding enzyme product or products, (b) allowing the dried bodily fluid or cell tissue sample to react with the eluent, incubation buffer and substrate or substrates for an adequate time and temperature, and (c) applying measuring means to the enzyme product to determine the activities of the lysosomal enzymes present.
Owner:GENZYME CORP
Methods and Devices for Treating Sleep Apnea and Snoring
ActiveUS20120227748A1Easy to transformForce is smallSuture equipmentsSurgical needlesTreatment sleepObstetrics
Methods and devices to prevent and / or treat breathing disorders (e.g., upper airway disorders) in mammals related to impaired airflow are described. Methods and devices apply force to soft tissue that avoids obstruction of airflow in the mammel's airway. Breathing disorders that are avoided by the methods and / or devices include apnea.
Owner:LINGUAFLEX INC
Noninvasive effective lung volume estimation
InactiveUS20050124907A1Minimal invasivenessLayered productsWithdrawing sample devicesEstimation methodsWashout
Methods for noninvasively measuring, or estimating, functional residual capacity or effective lung volume include obtaining carbon dioxide and flow measurements at or near the mouth of a subject. Such measurements are obtained during baseline breathing and during and shortly after inducement of a change in the subject's effective ventilation. The obtained measurements are evaluated to determine the amount of time required for exhaled carbon dioxide levels to return to normal—effectively an evaluation of carbon dioxide “washout” from the subject's lungs. Conversely, carbon dioxide and flow measurements may be evaluated to determine the amount of time it takes carbon dioxide to “wash in,” or reach peak levels within, the lungs of the subject following the change in the subject's effective ventilation. Apparatus for effective such methods are also disclosed.
Owner:RIC INVESTMENTS LLC
Fiber-optic sensing system
InactiveUS7196318B2Low costMinimal invasivenessRadiation pyrometryFluid pressure measurement by electric/magnetic elementsFiberGrating
The invention provides a fiber-optic sensing system, utilizing a fiber-grating-based sensor, for a physical parameter, e.g., a pressure or a temperature. Different kinds of fiber-grating-based sensors may be used for this purpose but in-fiber gratings such as Fiber Bragg Grating, Long Period Grating and Surface Corrugated Long Period Fiber Grating are particularly suitable. Due to the small size of the optical fiber and the fact that same fiber acts as the sensing element as well as the signal conducting medium, it is possible to install the sensor in a small diameter needle which is commonly used for medical diagnosis and treatment. As a result, when the fiber-optic sensing system of the invention is used for in-vivo measurement of a biological parameter, such a sensing needle can be used for different in-vivo pressure or temperature sensing applications without causing too much harm and discomfort to the subject tested.
Owner:YIP KIN MAN +1
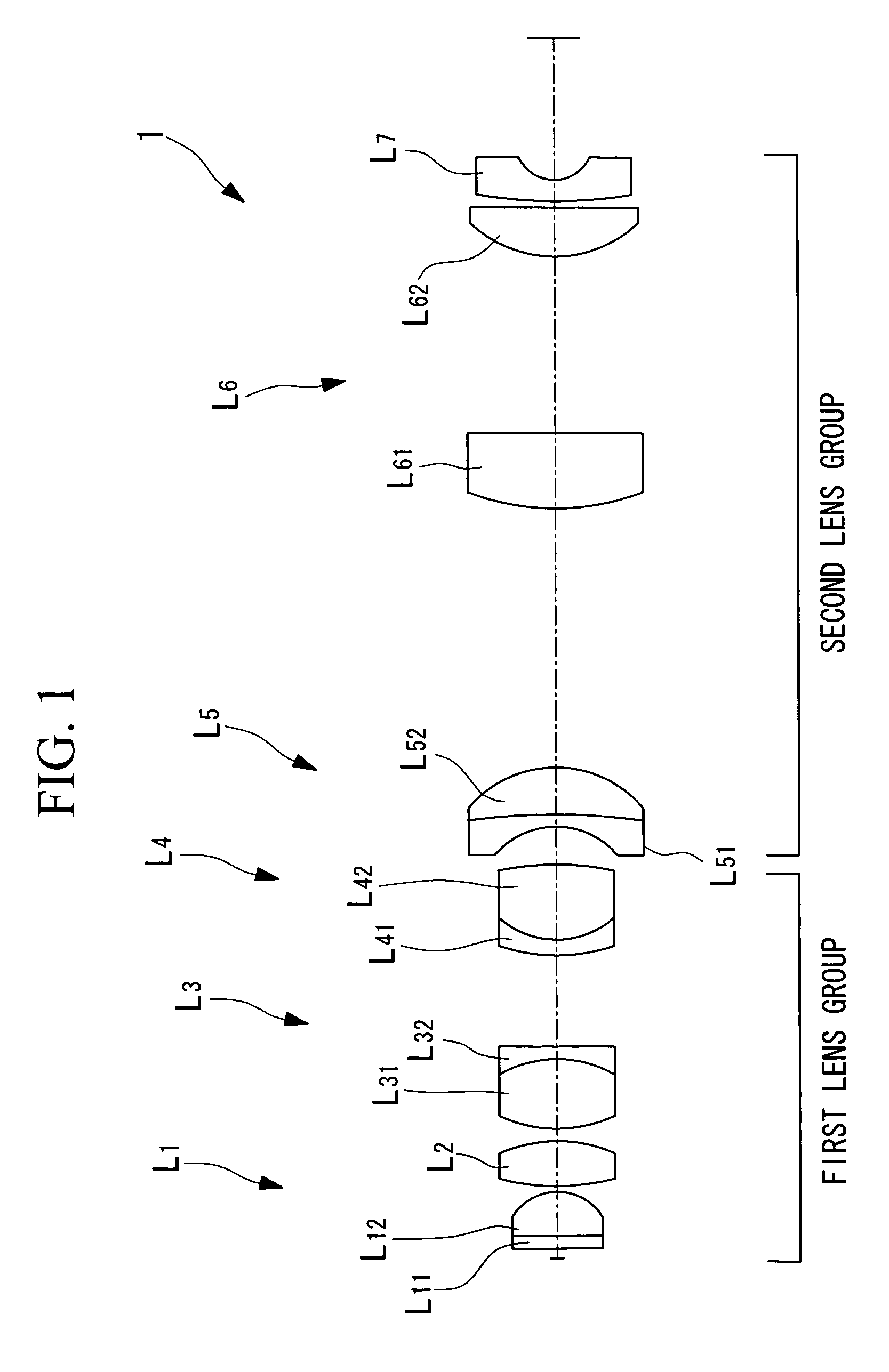
Liquid-immersion objective optical system
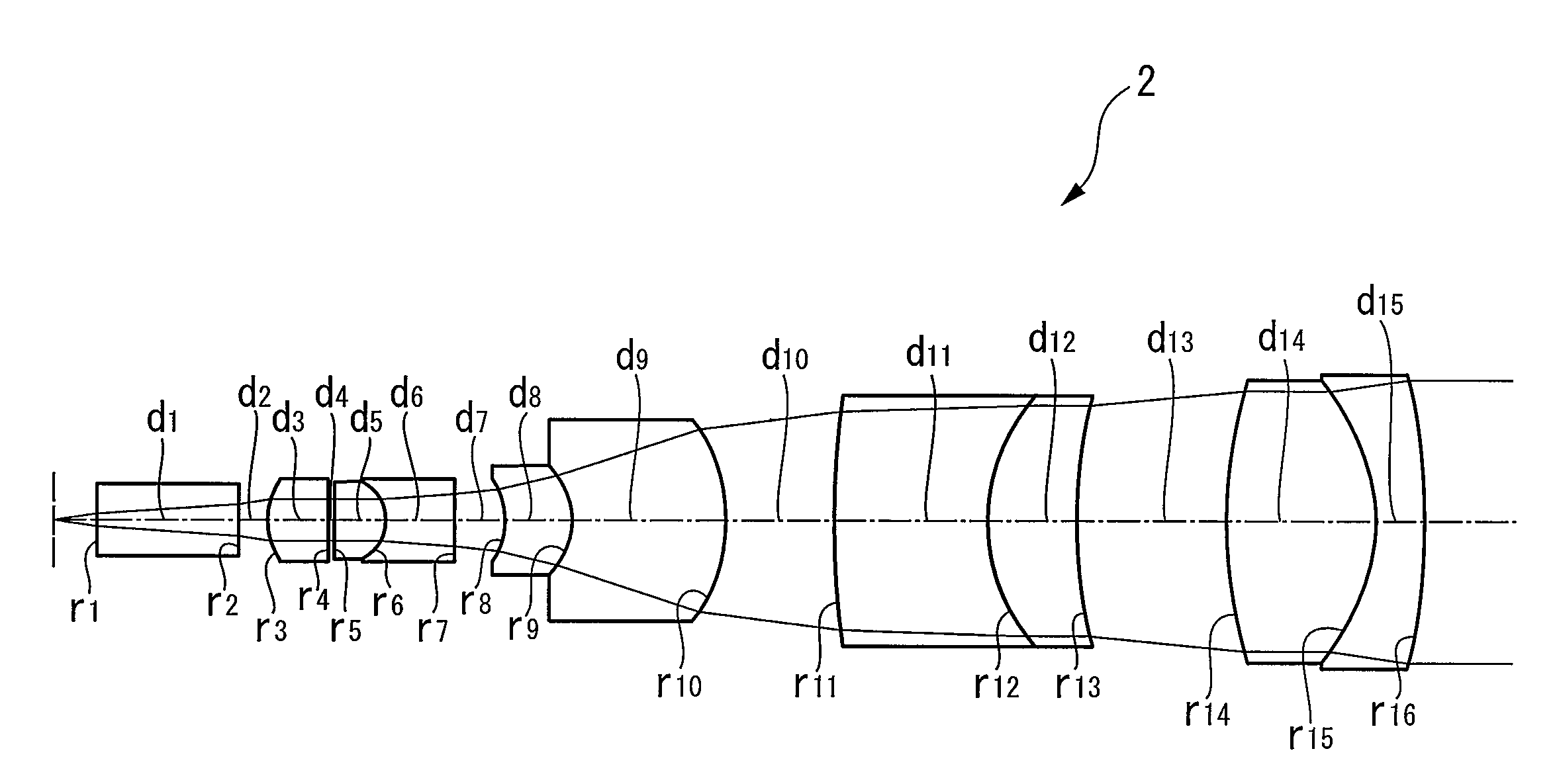
ActiveUS20060007558A1Minimal invasivenessHigh resolutionMicroscopesConditional expressionMagnification
The invention provides a liquid-immersion objective optical system including a first lens group and a second lens group. The first lens group includes a first lens component having positive refractive power, an object side thereof being a flat surface and a convex surface thereof facing an image-plane side; a second lens component having positive refractive power, a convex surface thereof facing the image-plane side; a third lens component having positive refractive power as a whole, formed by cementing a biconvex positive lens and a negative lens; and a fourth lens component formed by cementing a negative lens and a biconvex positive lens. The second lens group includes a fifth lens component formed by cementing a lens having a concave surface facing the object side and a lens having a convex surface facing the image-plane side; a sixth lens component having positive refractive power; and a seventh lens component having negative refractive power. The focal length F1 of the entire first lens group, the magnification M of the objective optical system, the distance LT between the object plane and the image plane, and the distance LG between the object side of the first lens component L1 and the image side of the fourth lens component L4 satisfy the following conditional expressions: 0.2<|M·F1 / LT|<0.45 0 / 2<|LG1 / LT|<0.4
Owner:EVIDENT CORP
Therapeutic delivery device
ActiveUS9113950B2Minimal invasivenessPrecision therapyInfusion syringesSurgical needlesIntervertebral discEngineering
Methods and device are provided for delivery of biologics to an intervertebral disc. Device described herein include a needle assembly and handle assembly that optimize biologic delivery parameters to a target location within a disc. The needle assembly includes the capability of advancing into the disc at pre-configured pathways that allow for minimized damaged and maximized positioning of the biologic.
Owner:REGENEXX LLC
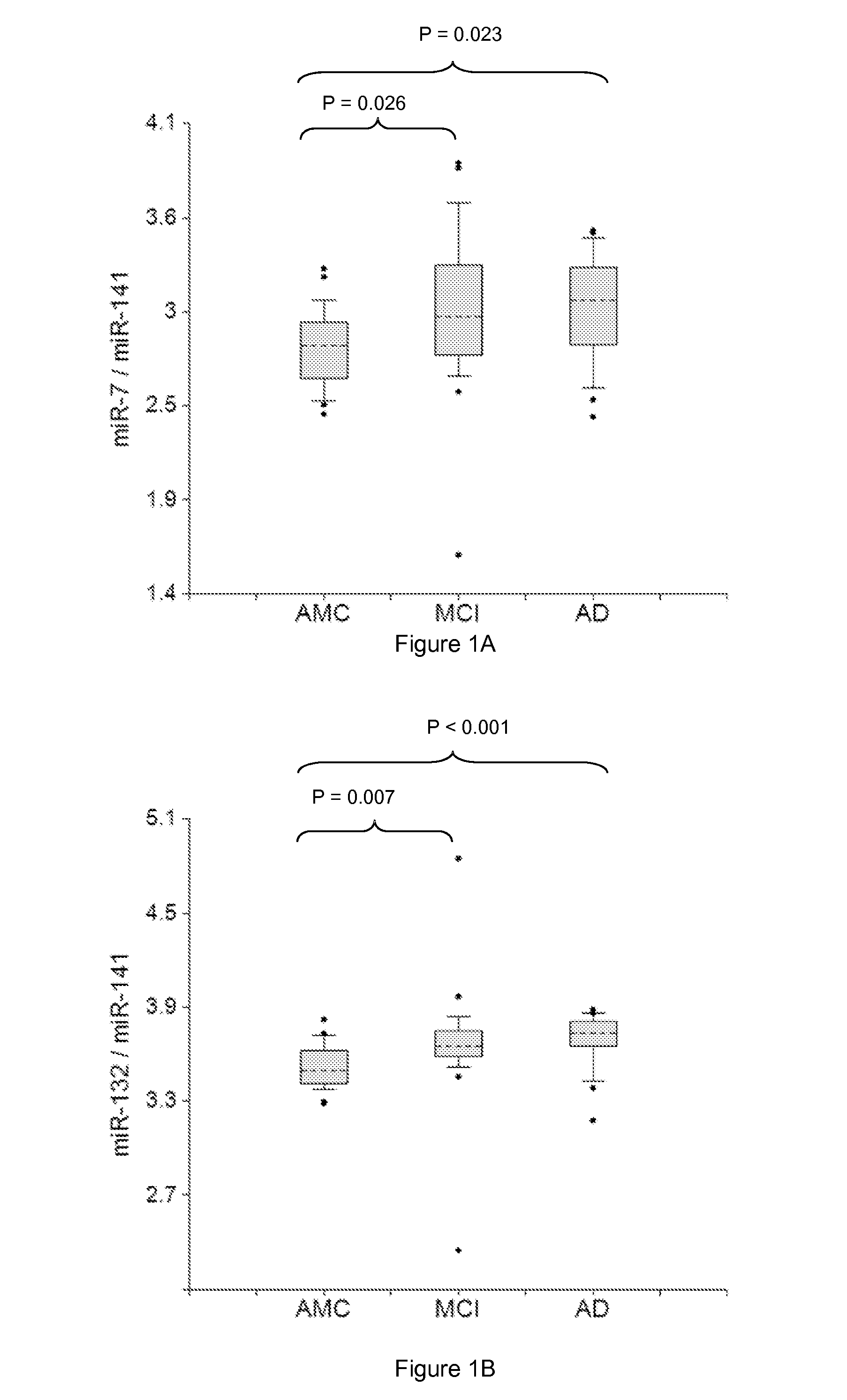
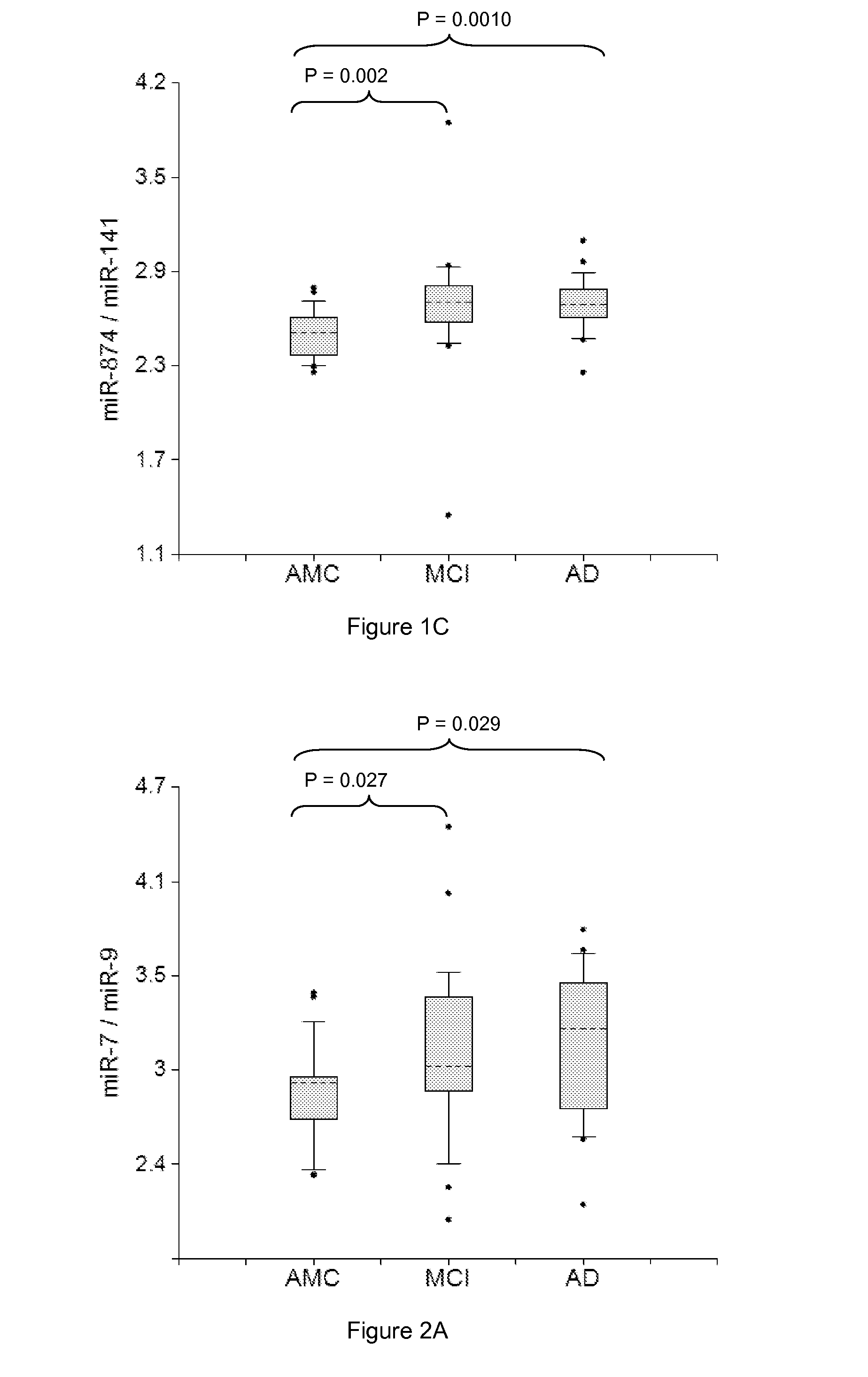
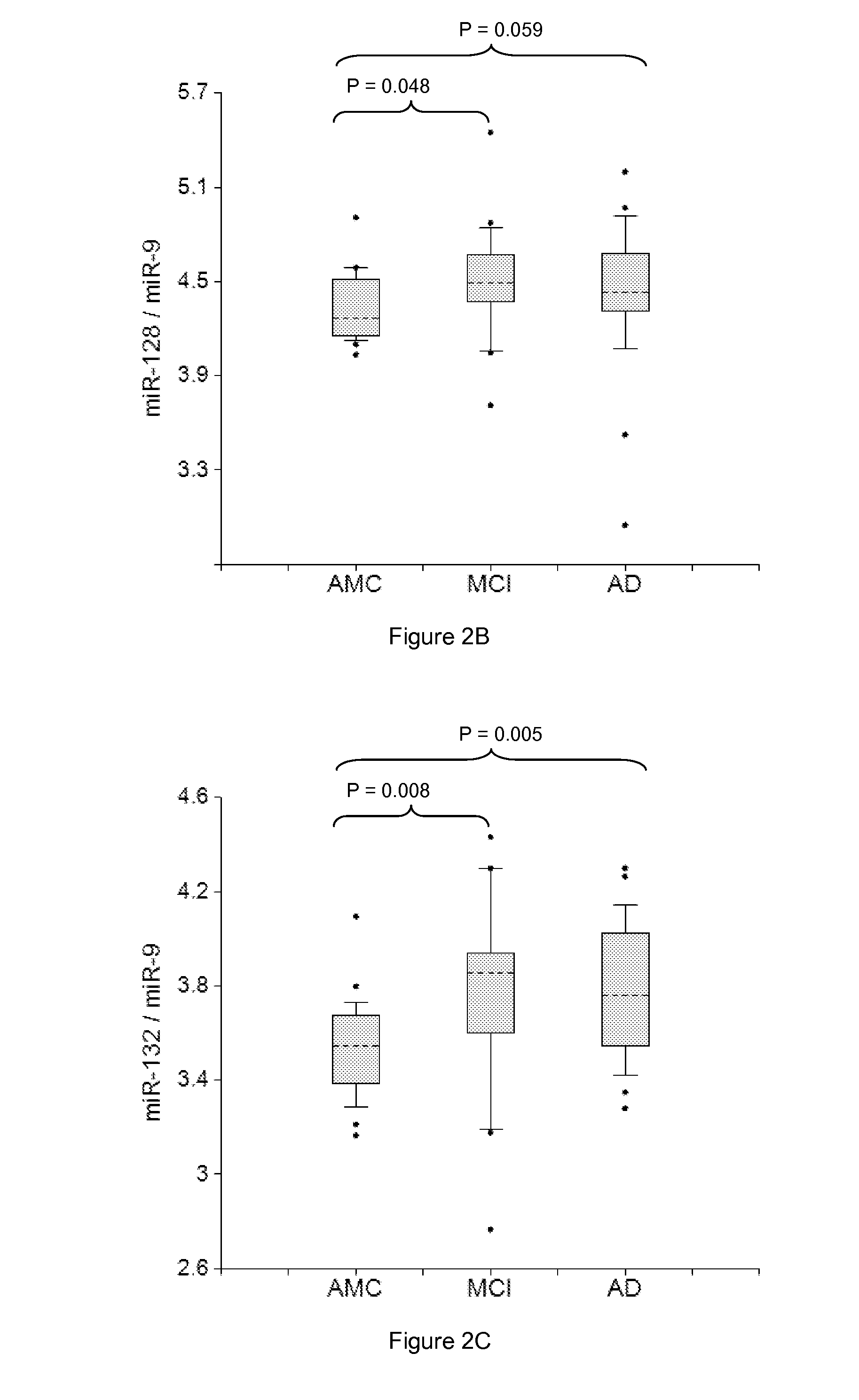
miRNA-BASED UNIVERSAL SCREENING TEST (UST)
ActiveUS20140256562A1Strong specificityEasy diagnosisNervous disorderNucleotide librariesOrgan systemBody fluid
Described are methods for early noninvasive or minimally invasive detection of pathological changes in organ systems / organs / tissues / cells by quantifying organ system- / organ- / tissue- / cells type-enriched miRNA in bodily fluids.
Owner:DIAMIR
Methods
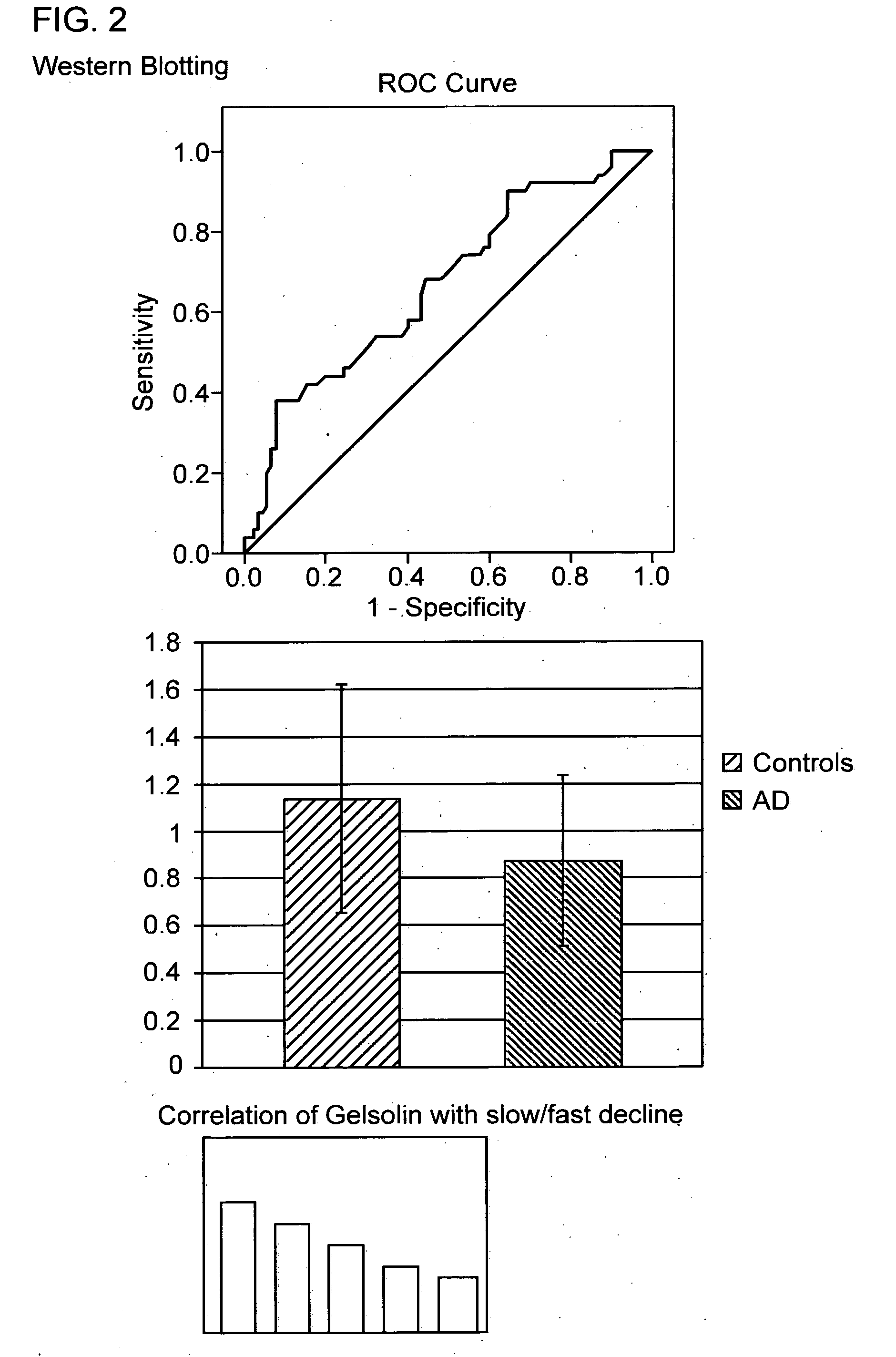
InactiveUS20120071337A1Reliable indicationSuperior methodPeptide librariesPeptide/protein ingredientsReference sampleDisease
The invention provides a method for aiding the diagnosis or prognostic monitoring of Alzheimer's disease in a subject, said method comprising; providing a sample of blood obtained from said patient; assaying the amount of gelsolin present in said sample; comparing the amount of gelsolin present in said sample to a reference amount of gelsolin present in a sample from a healthy subject, wherein detection of a gelsolin level in the sample from said patient which is lower than the gelsolin level in the reference sample indicates an increased likelihood of Alzheimer's disease in said patient. Other markers are C1 protease inhibitor and ceruloplasmin. Both blood samples and tissue samples have been investigated.
Owner:ELECTROPHORETICS LTD +1
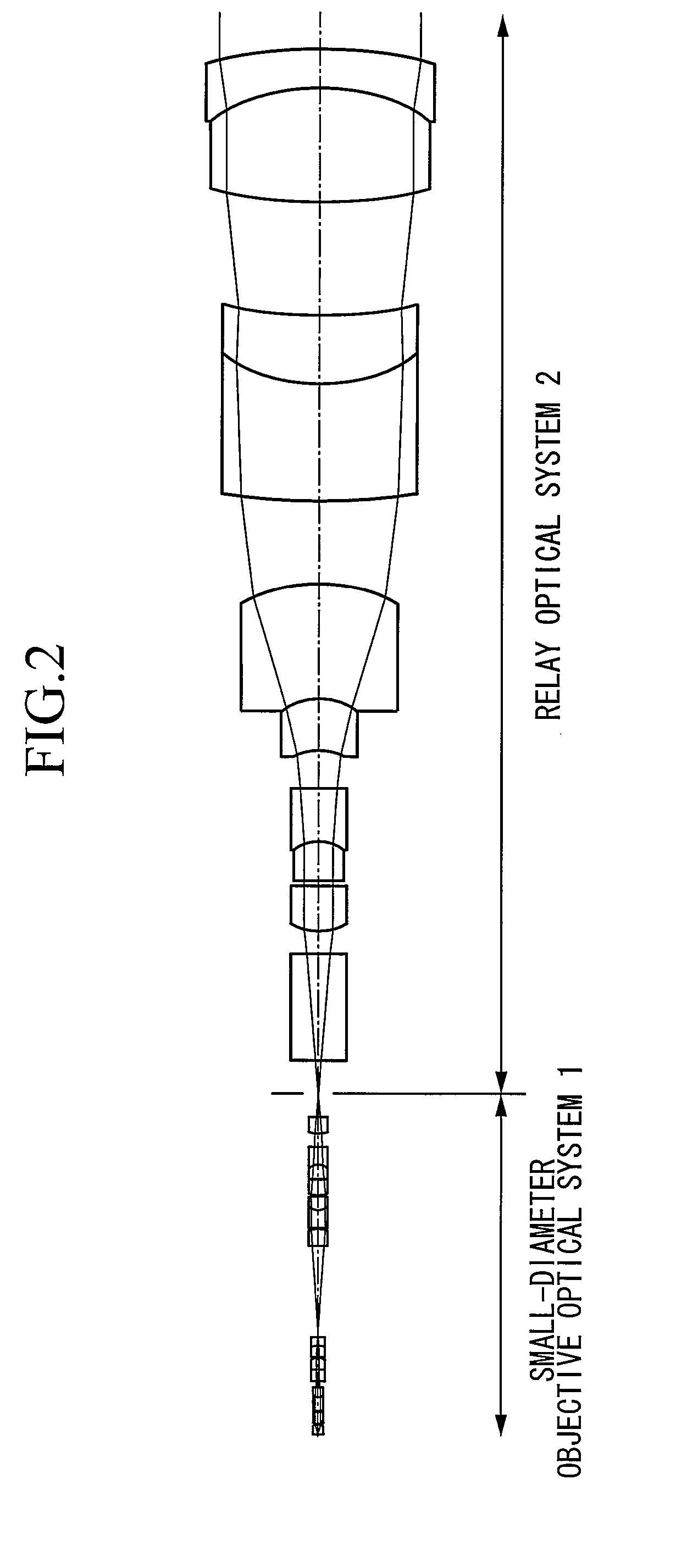
Small-diameter objective optical system
Reducing the outer diameter and effectively correcting various aberrations realizes a small-diameter objective optical system suitable for in vivo observation with a high numerical aperture. The invention provides a small-diameter objective optical system comprising, in order from an object plane a first lens group with positive refractive power, including at least one plano-convex lens whose convex surface faces an image plane; a second lens group with positive refractive power, including at least one concave lens; and a third lens group including a cemented lens of which a cemented surface has negative refractive power. The focal length of the third lens group is larger than the focal length of the first lens group.
Owner:EVIDENT CORP
Liquid-immersion objective optical system
The invention provides a liquid-immersion objective optical system including a first lens group and a second lens group. The first lens group includes a first lens component having positive refractive power, an object side thereof being a flat surface and a convex surface thereof facing an image-plane side; a second lens component having positive refractive power, a convex surface thereof facing the image-plane side; a third lens component having positive refractive power as a whole, formed by cementing a biconvex positive lens and a negative lens; and a fourth lens component formed by cementing a negative lens and a biconvex positive lens. The second lens group includes a fifth lens component formed by cementing a lens having a concave surface facing the object side and a lens having a convex surface facing the image-plane side; a sixth lens component having positive refractive power; and a seventh lens component having negative refractive power. The focal length F 1 of the entire first lens group, the magnification M of the objective optical system, the distance LT between the object plane and the image plane, and the distance LG between the object side of the first lens component L 1 and the image side of the fourth lens component L 4 satisfy the following conditional expressions: <?in-line-formulae description="In-line Formulae" end="lead"?>0.2<|M.F1 / LT|<0.45<?in-line-formulae description="In-line Formulae" end="tail"?> <?in-line-formulae description="In-line Formulae" end="lead"?>0 / 2<|LG1 / LT|<0.4.<?in-line-formulae description="In-line Formulae" end="tail"?>
Owner:EVIDENT CORP
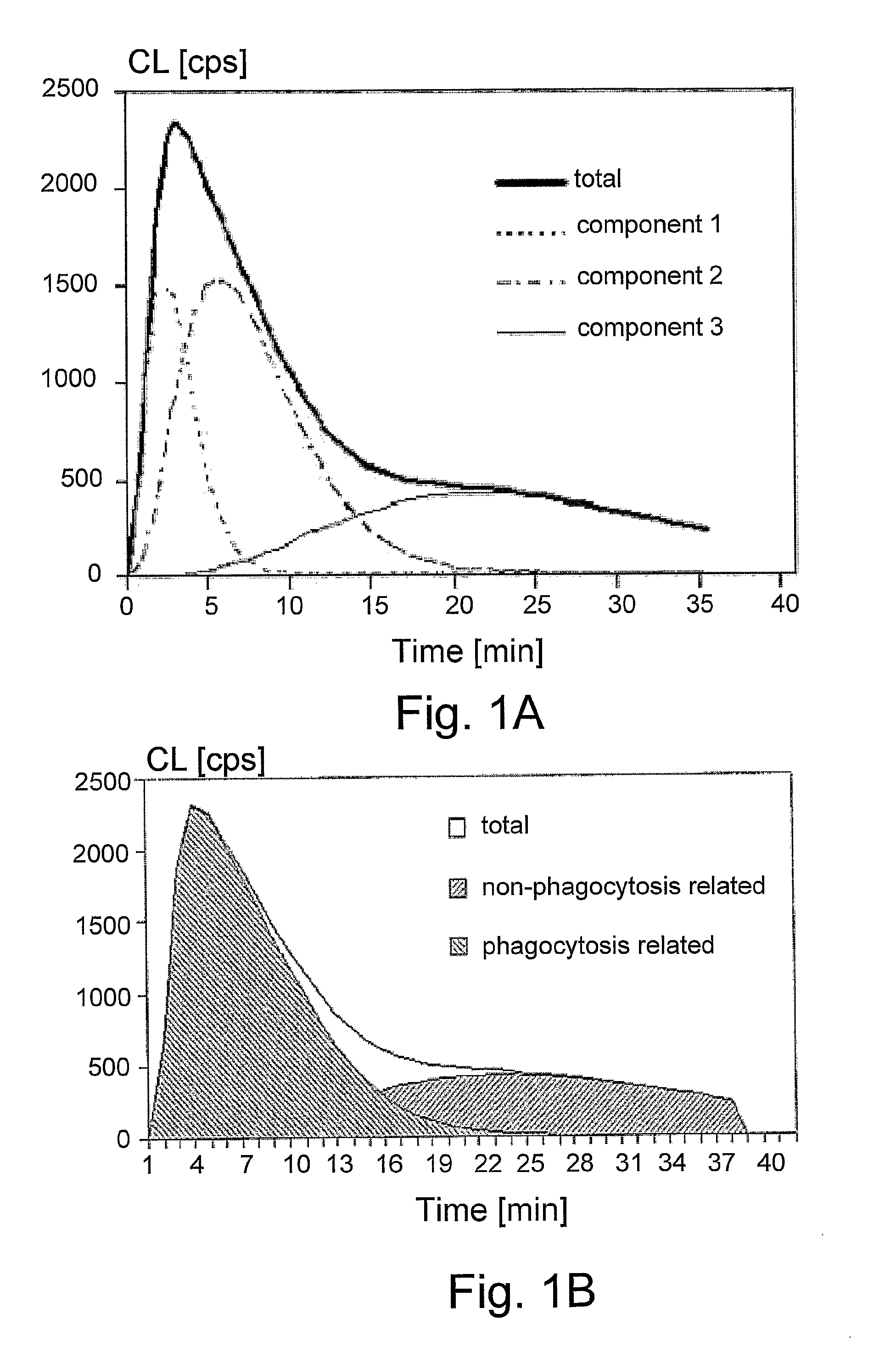
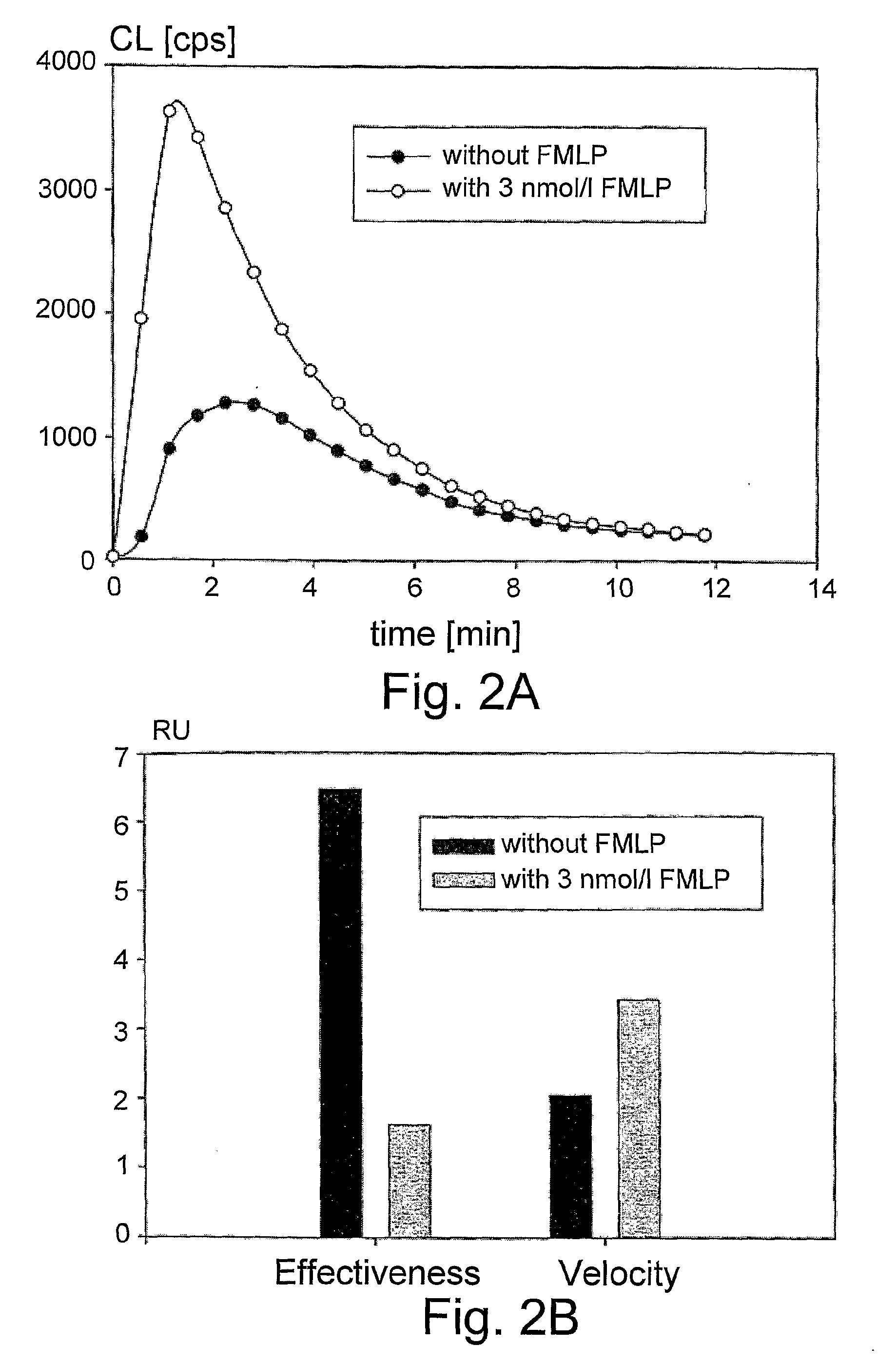
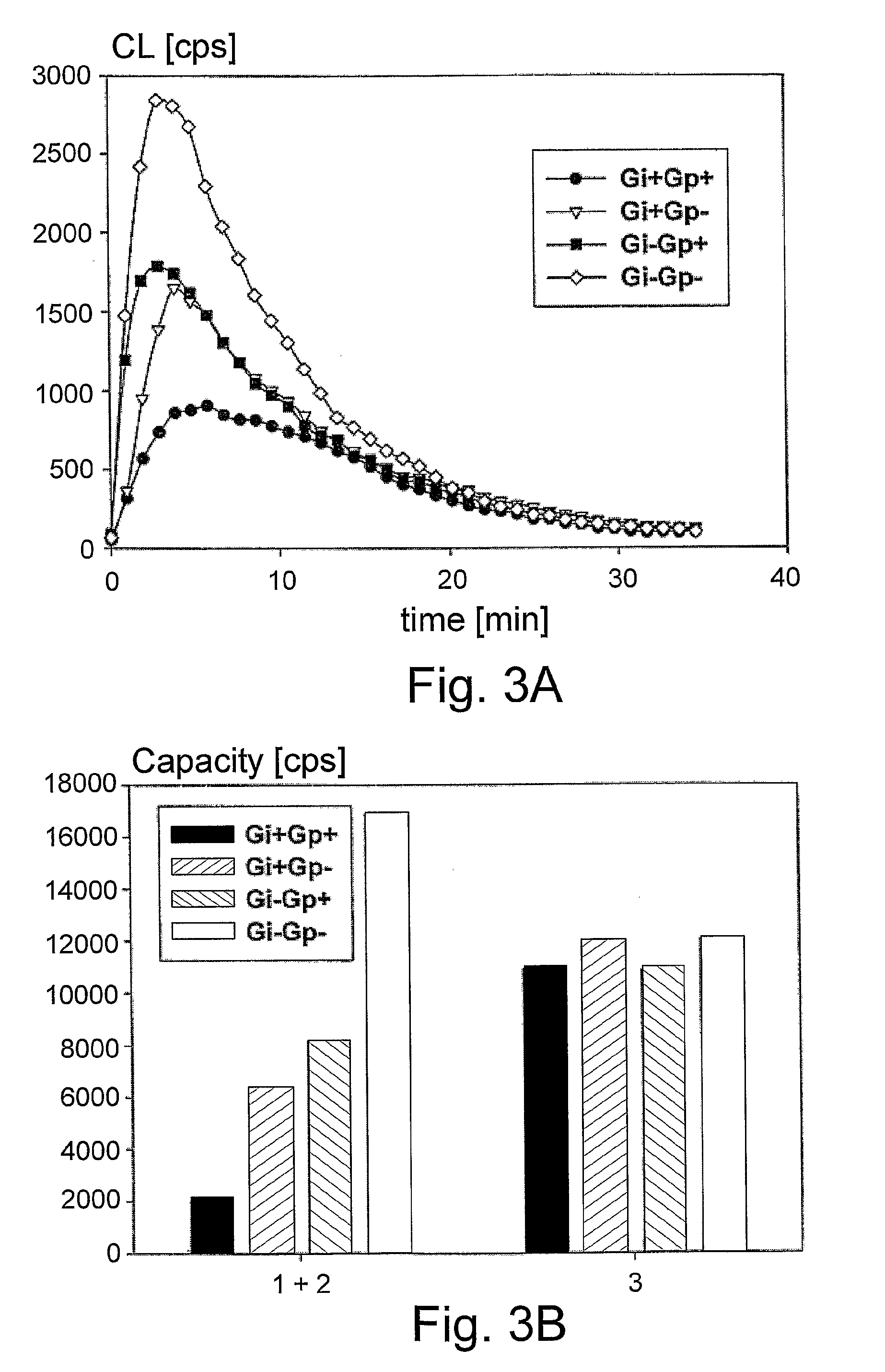
Chemiluminescent Method and Device for Evaluating the In Vivo Functional State of Phagocytes
InactiveUS20090053751A1High sensitivityMinimal signal lossBioreactor/fermenter combinationsBiological substance pretreatmentsPhagocyteOxygen
A method of assessing the in vivo state of phagocytes in a patient, possibly indicating diagnostically important states such as inflammation or infection, which method utilizes chemiluminescent (CL) light emitted during the reaction in vitro between a CL substrate and the reactive oxygen species (ROS) formed in a fluid sample obtained from the patient. The measurement is performed in two or more portions of the sample, with stimulated phagocytes affected by one or more priming agents which shift the functional state of the phagocytes, providing a plurality of measurements, which are analyzed so as to distinguish intracellular and extracellular contributions to the CL kinetics. The results are compared with a range of control measurements performed with patients suffering from various diagnostic conditions.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Device and system for measuring forces
InactiveUS20070181789A1Increase the number ofLess demanding mechanical toleranceRadiation pyrometryForce measurementEngineeringOptical transducers
Optical transducers are provided for detecting forces such as, for example rotational and transversal forces acting on them, wherein, for the purpose of detecting said forces, optical signals are transmitted through an optical path defined by optical fibers. Moreover, optical means are provided, adapted to modify the transmission of the optical signals through the optical path, as a result of forces acting on them. The resulting optical signals exiting the transducer are then used for the purpose of detecting the forces acting on the transducer.
Owner:IST SUPERIORE MARIO BOELLA +1
Implantable systems and stents containing cells for therapeutic uses
InactiveUS9788978B2Eliminate needMinimal invasivenessStentsFiltering accessoriesPeritoneal cavityBlood vessel
Owner:ROJO NICHOLAS A
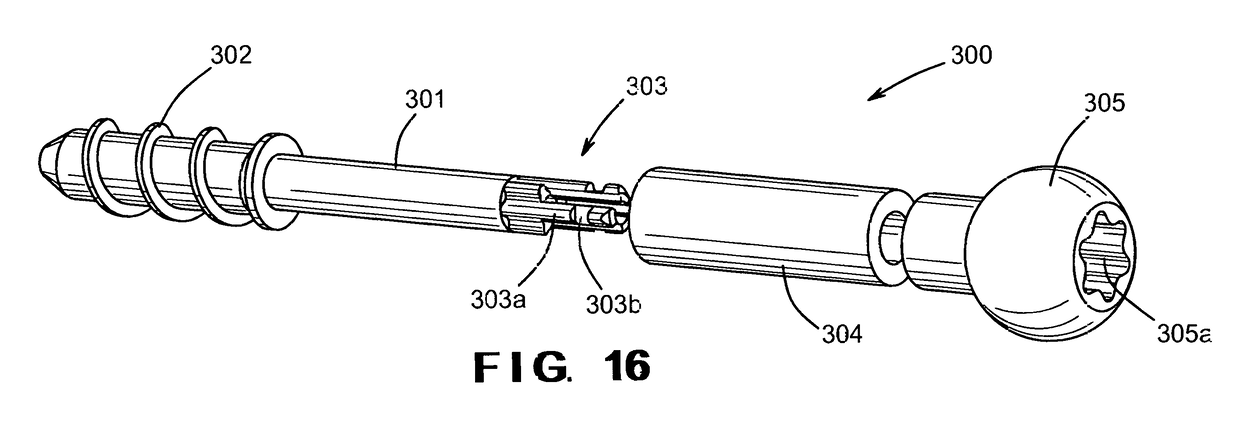
Bioactive fusion device
ActiveUS9808299B2Reduce amountLower medical costsInternal osteosythesisSpinal implantsDistal portionEngineering
In a first broad aspect, there is provided herein a bioactive device and system for fusion between two bones, two parts of a bony joint, or a bony defect, such as of the spine. The fusion device includes a screw having a head and a threaded shaft. The fusion device also includes a bone dowel having an internal bore of which at least a distal portion is threaded to engage the threads of the screw shaft. The bone dowel is made of a bone-like, biocompatible, or allograft material to provide a layer of bone-like, biocompatible, or allograft material between the screw and the spinal bone. The device is generally coaxial and is further described in the drawings and description herein.
Owner:UNIVERSITY OF TOLEDO
Biocompatible composition for replacing/regenerating tissues
InactiveCN101189035AMinimal invasivenessPharmaceutical delivery mechanismTissue regenerationCross-linkVolumetric Mass Density
A biocompatible composition for replacing / regenerating articular tissues, in particular tissues of the nucleus pulposus but also not articular tissues such as bony tissues, providing a hydrogel matrix comprising a first and a second natural polymer, cells adapted to regenerate the tissue of the disc core, a cross linking agent suitable to cross-link at least one of said natural polymers in situ, thus giving to the biocompatible composition an appropriate resistance, and molecules adapted to stimulate the growth and / or the differentiation of cells, wherein one of the two natural polymers is sodium alginate. In a particular exemple, the above described composition before the cross-linking step has a starting density such that it is injectable. In particular, the cross linking action on sodium alginate is caused by a cross linking agent contained in biodegradable polymeric particles, released in a controlled way from natural polymers, for example gelatin, or starting from synthetic polymers, for example polylactic acid or copolymers of acid lactic-glycolic acid.
Owner:朱塞佩·卡尔沃萨
Bioactive fusion device
ActiveUS9743961B2Reduce amountLower medical costsInternal osteosythesisFastenersBiomedical engineeringBiological activity
Owner:UNIVERSITY OF TOLEDO
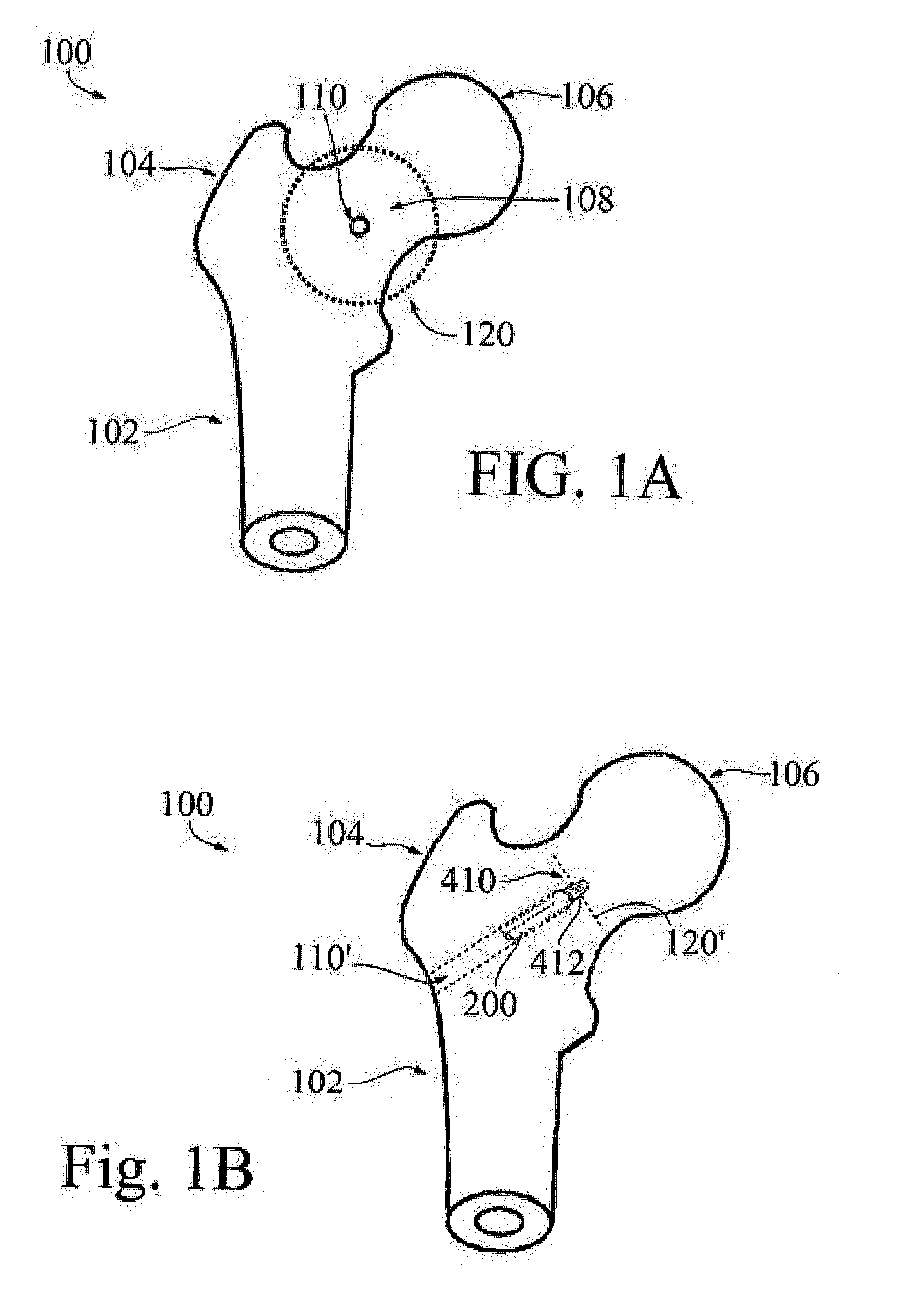
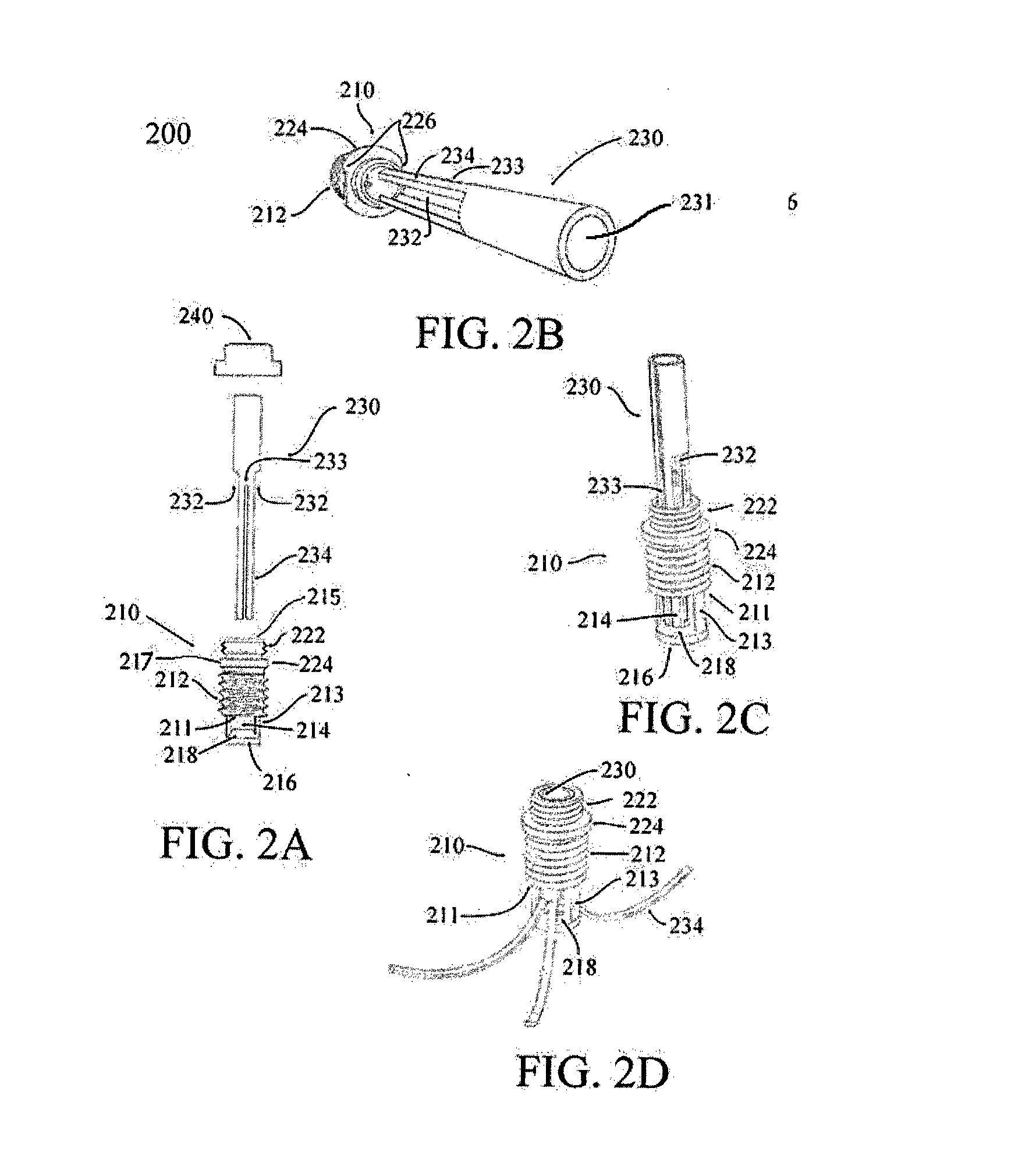
Hip implant
ActiveUS20150250506A1Prevent rotationMinimal invasivenessInternal osteosythesisJoint implantsHip joint implantationDistal portion
A system and method for forming a hip implant offering minimal invasiveness is described. The system comprises means for positioning the implant under optical and X-ray control, an implant and an applicator for screwing and expanding the implant. The implant includes an insert body and an expansion tube with cut-outs and slits defining legs. The legs expand radially through windows in the insert body when the expansion tube is pushed against the sloped distal portion of the insert body. The expansion process may be ensured by using an anti-rotation device to prevent the insertion tube from rotating within the insert body. Applicator and implant are provided with means preventing cold welding during the implantation process.
Owner:MJP INNOVATIONS INC
Method for assaying the activity of lysosomal enzymes
InactiveUS20090325206A1Inexpensive and simple for determinationSimple and expedite sample collectionMicrobiological testing/measurementLysosomeGlucuronidase
A method, and associated kit, for assaying the activity of lysosomal enzymes present in dried bodily fluids and cell tissue samples, such as α-L-iduronidase, β-D-galactosidase, β-D-glucosidase, chitotriosidase, total α-D-galactosidase and α-D-galactosidase A, hexosaminidase A and B, α-D-mannosidase, β-D-mannosidase, α-L-fucosidase, N-acetyl-α-galactosaminidase, arylsulfatases, sphingomyelinase, β-galactocerebrosidase, iduronate-2-sulfatase and β-D-glucuronidase. The method includes: (a) combining with a dried bodily fluid or cell tissue sample containing at least one type of lysosomal enzyme: (1) an eluent, (2) an incubation buffer and (3) a substrate or substrates capable of reacting with the assayed lysosomal enzymes and producing their corresponding enzyme product or products, (b) allowing the dried bodily fluid or cell tissue sample to react with the eluent, incubation buffer and substrate or substrates for an adequate time and temperature, and (c) applying measuring means to the enzyme product to determine the activities of the lysosomal enzymes present.
Owner:GENZYME CORP
Nail Clipper with Thin Angled Wrap-around Cover-Guard
ActiveUS20220031041A1Security solutionMaximum protectionManicure/pedicureArchitectural engineeringKnife blades
As shown in one preferred embodiment, a two-blade vice-type nail clipper comprising a thin safety-cover-guard with ergonomic upward angle and wrap-around safety edges, that is permanently mounted just below said nail clipper's lower cutting blade and that follows or mirrors the shape of said nail clipper's lower cutting surface, including wrapping around the sharp sides, corners and edges of said lower cutting surface, so as to provide an industry advancing level of nail cutting safety with minimal invasiveness.
Owner:DAUGHERTY CHARLES RICHERD
Interrupting the life cycle of sperm
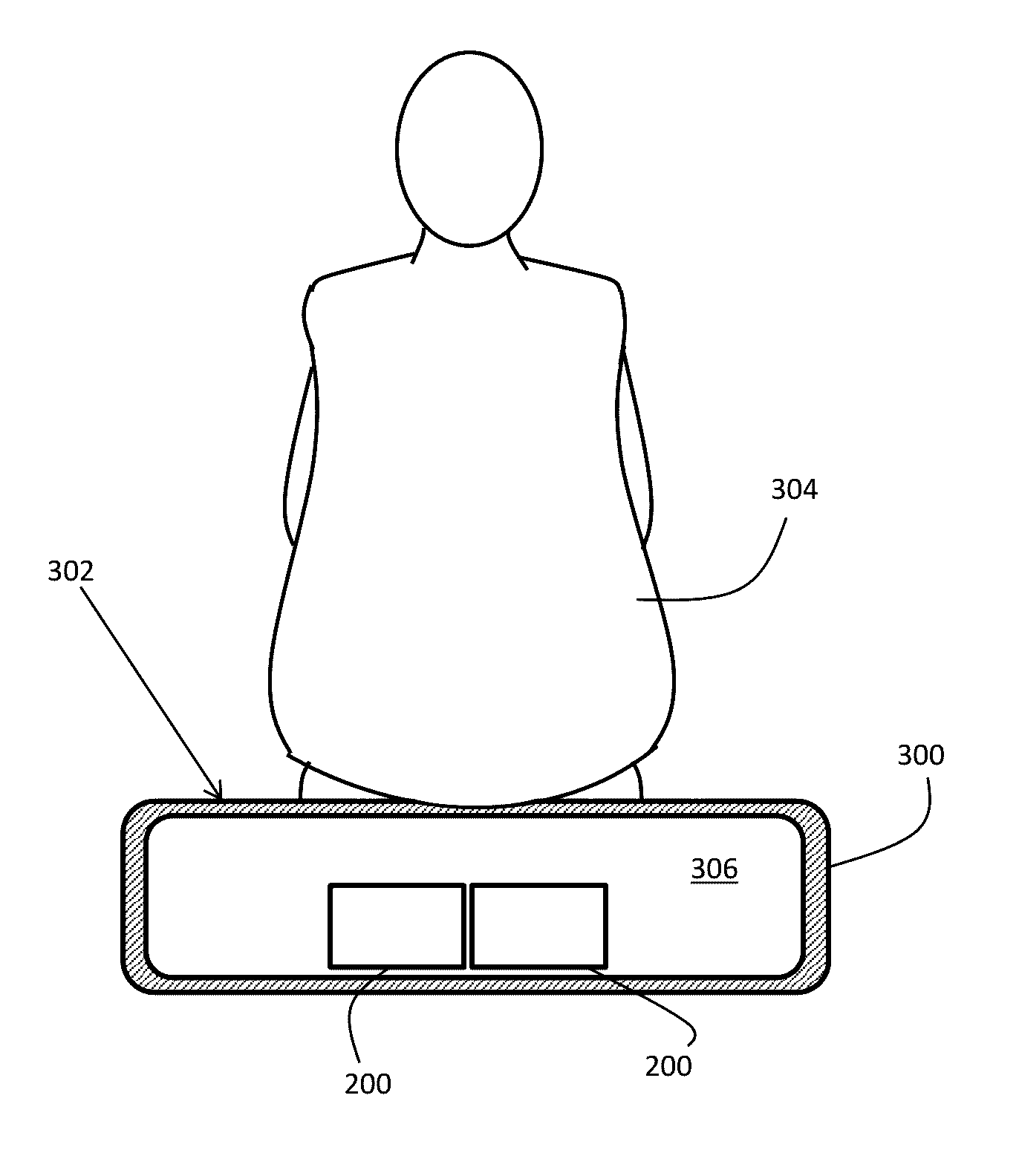
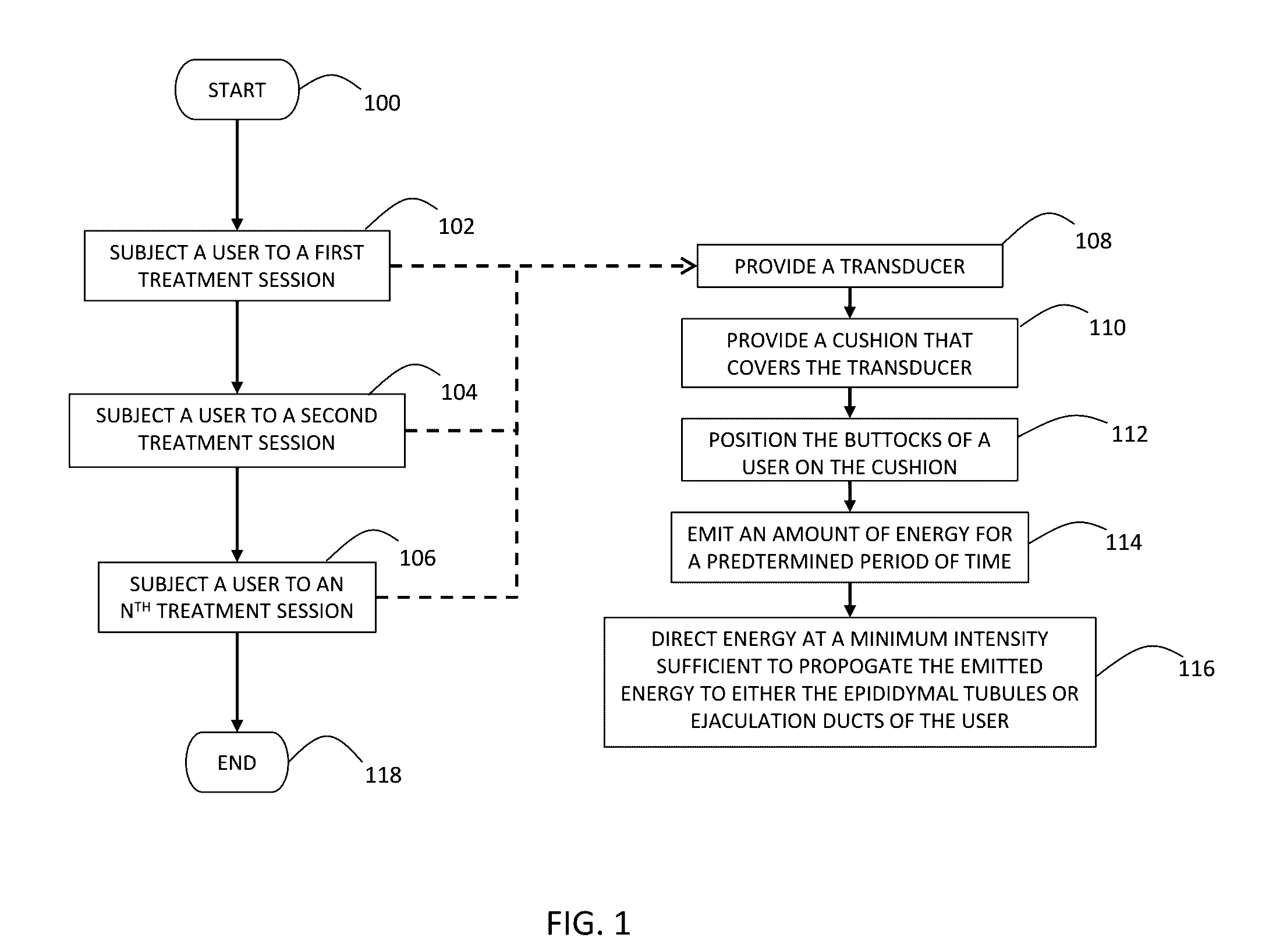
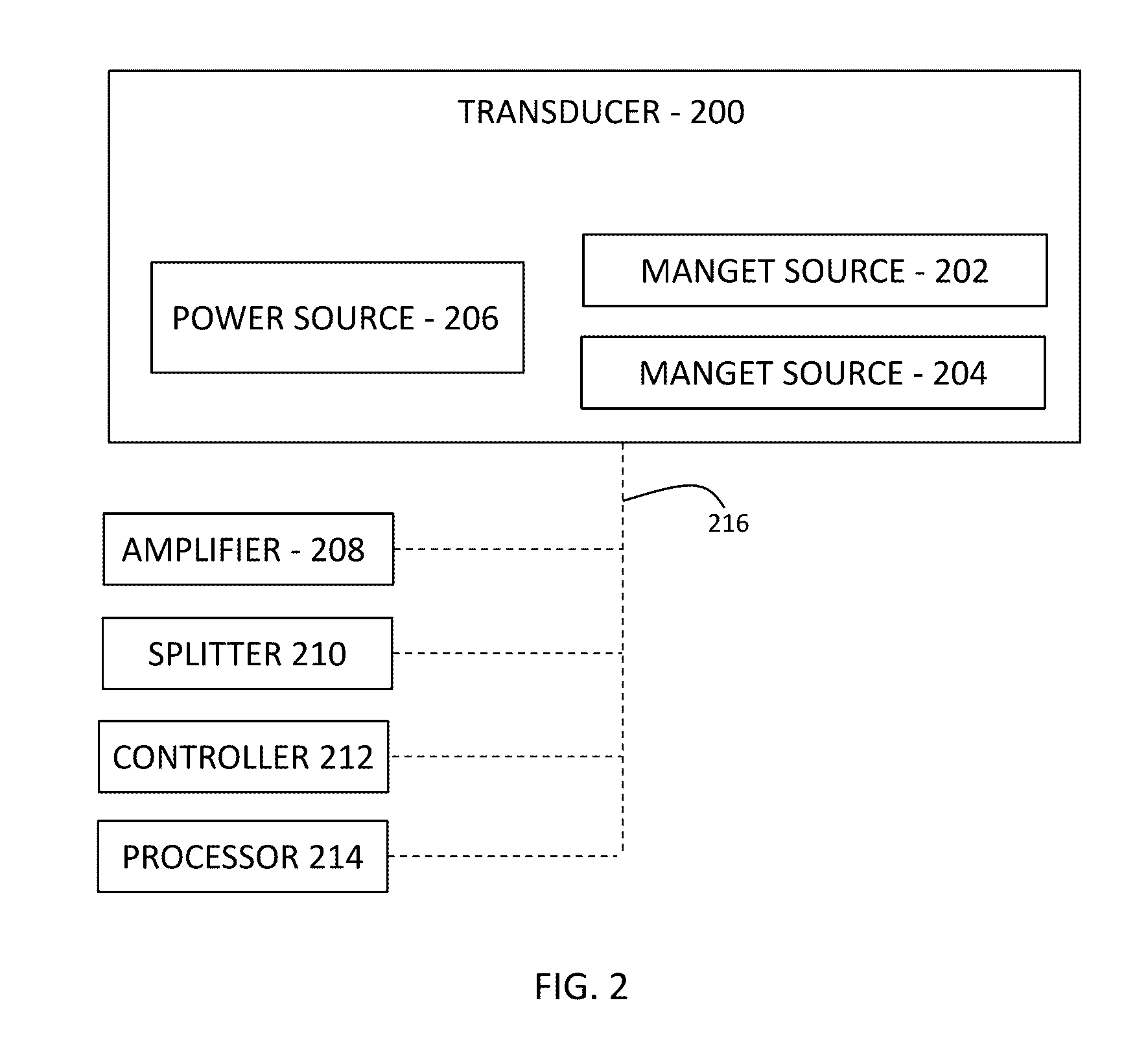
ActiveUS20160324682A1Minimal invasivenessUltrasound therapyMale contraceptivesReproductive systemEngineering
A method of interrupting the life cycle of sperm that includes subjecting a user to a first and a second treatment session in a male birth-control cycle. The first treatment session including a mature-sperm inhibition step of emitting, through at least one transducer, an amount energy for a predetermined period of time that is directed to the entire male reproductive system including but not limited to at least one of an epididymal tubules and an ejaculation ducts of the user, via a constant coupling configuration of the transducer with the user, to render a plurality of sperm transported therein to be immotile. The second treatment session includes subjecting the user to the mature-sperm inhibition step before a sperm-maturation time whereby sperm carried by the user are in a mature state.
Owner:SOUND TECH TRANSFER LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com