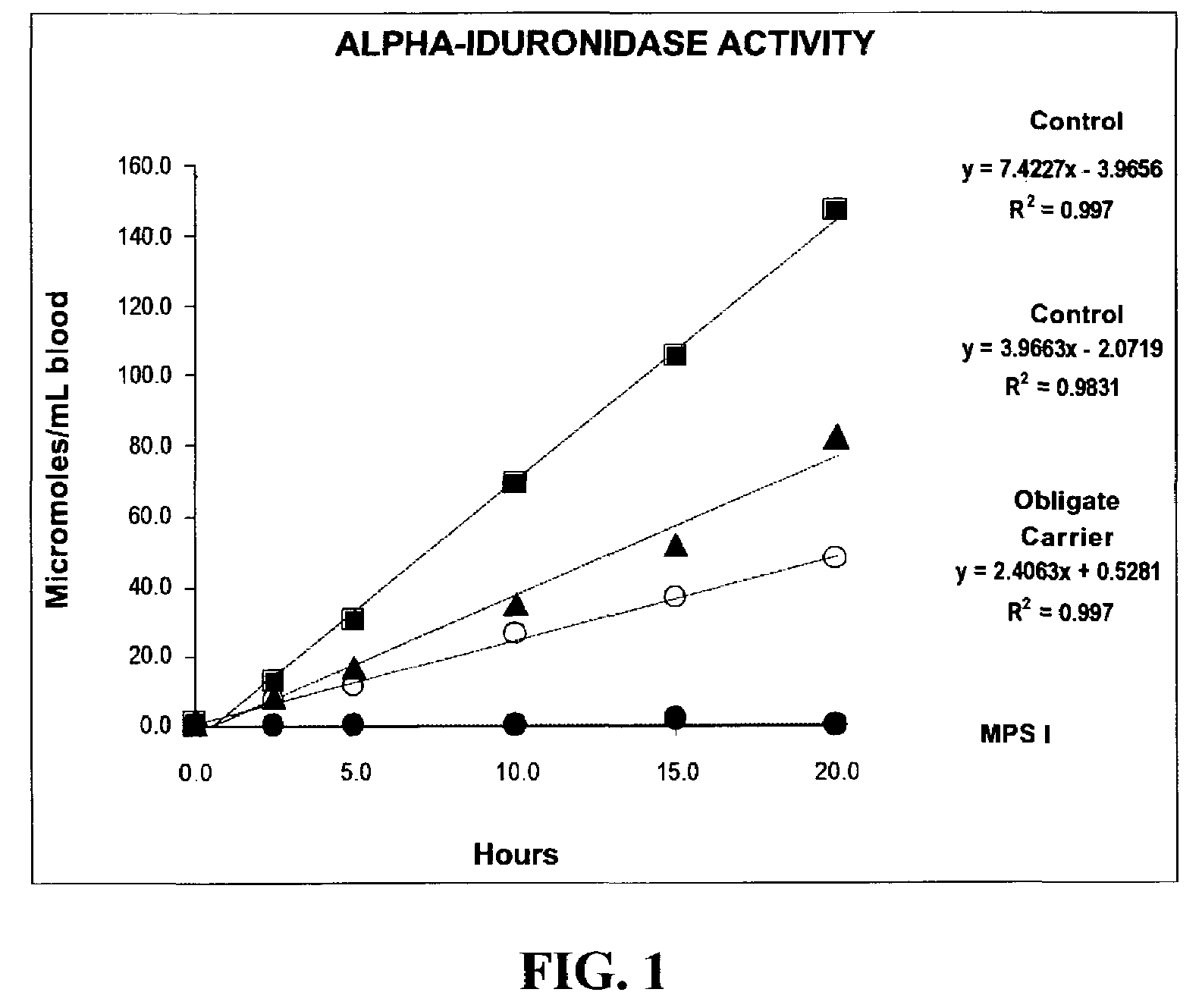
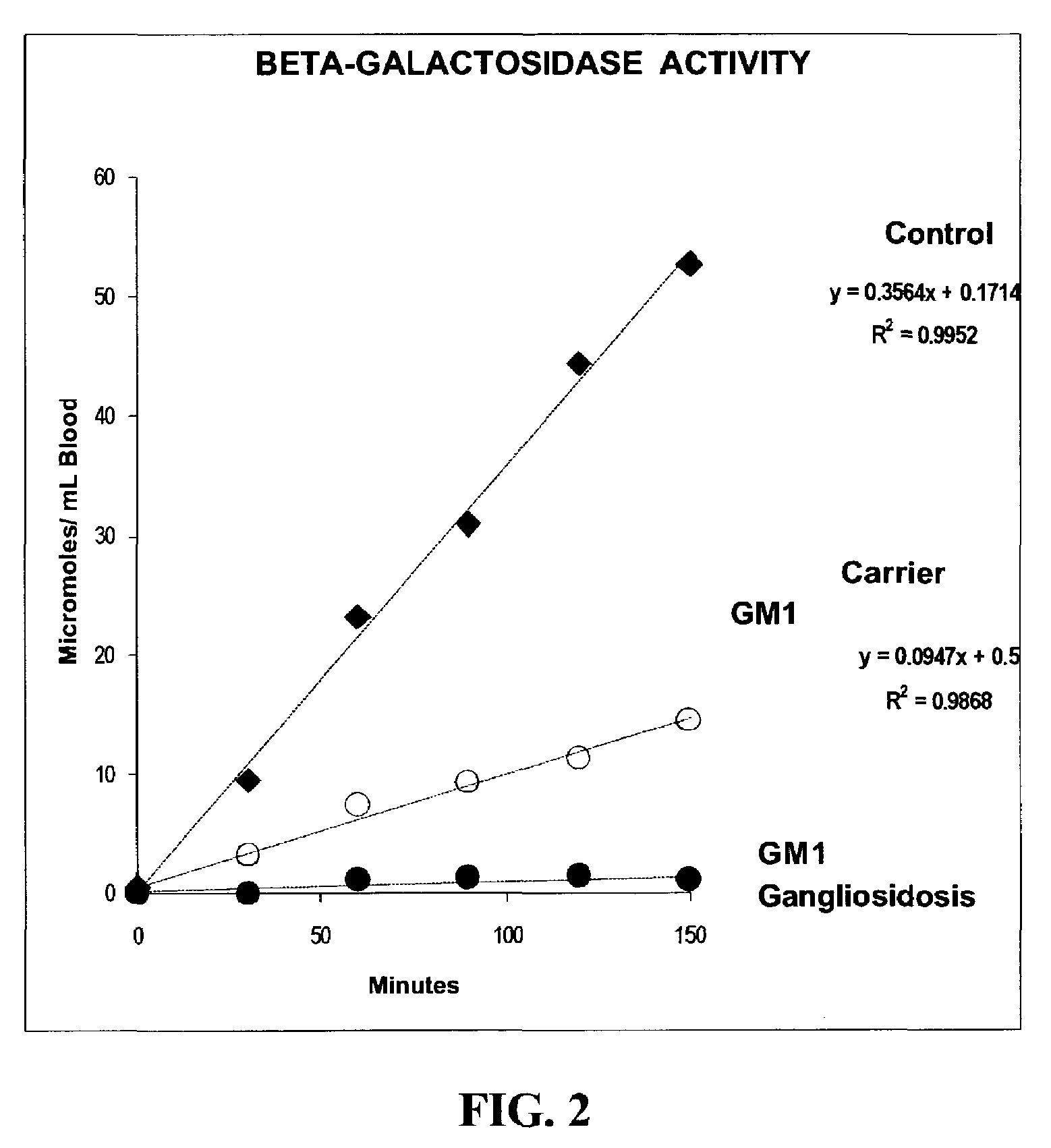
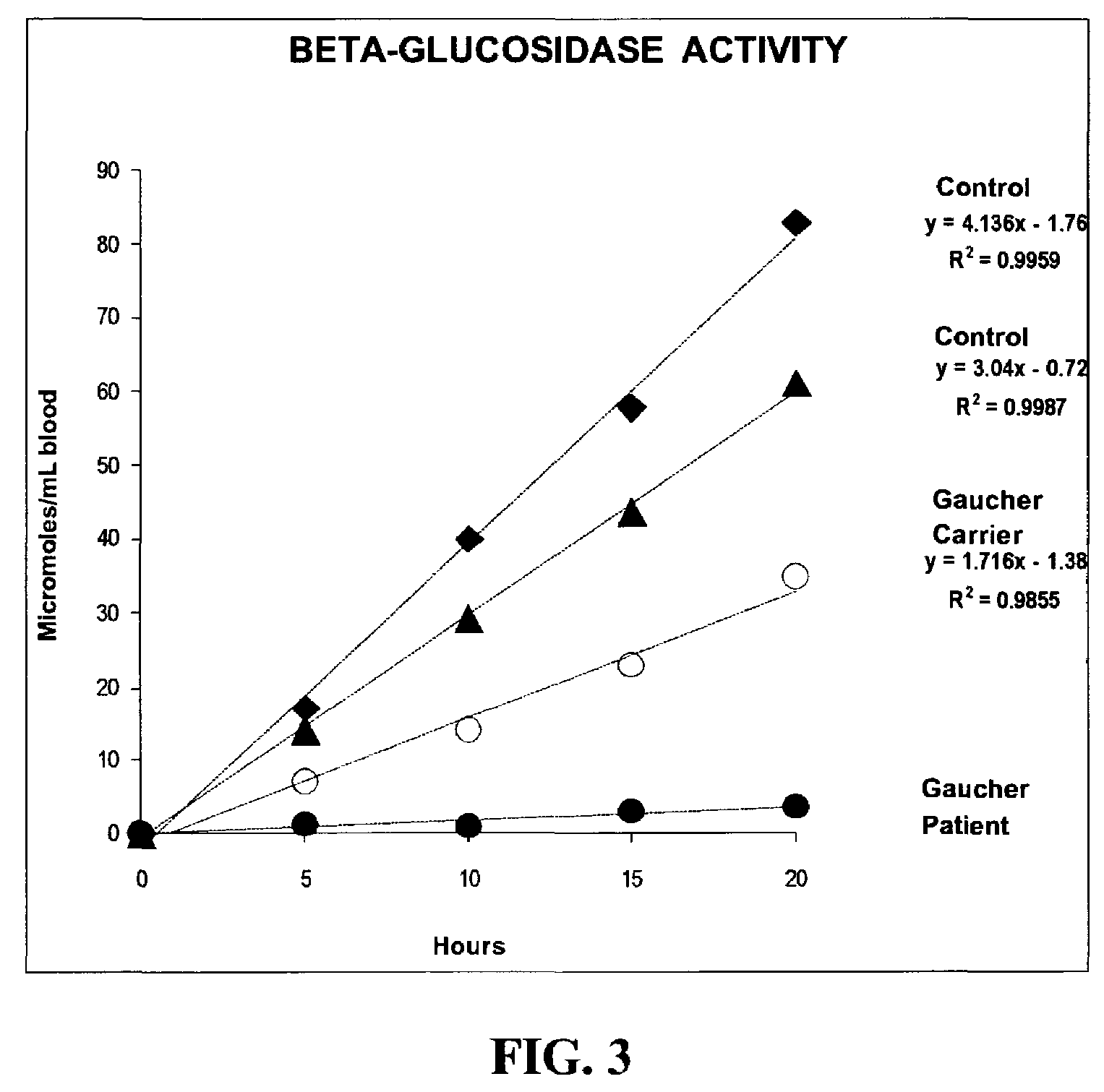
Method for assaying the activity of lysosomal enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Dried Blood Samples
[0101]A drop of blood obtained by venepuncture was spotted on filter paper (Schleicher and Schuell N° 903, Keene, N.H., USA) and allowed to dry at room temperature (22° C.) overnight on a flat non-absorbing surface. The dried blood spots on filter paper were stored on plastic bags at 4° C. until analysis.
[0102]A sample for analysis was prepared by punching out a 3 mm-diameter circle (about 5.5 μl of whole blood) with a standard paper punch from the dried blood spots on the filter paper. A sample for analysis was prepared by punching out a 1.5 mm-diameter circle (about 2 μl of whole blood) with a standard paper punch from the dried blood spots on the filter paper.
[0103]This protocol was also performed by utilizing a heelprick finger to obtain the blood.
example 2
Preparation of Dried Chorionic Villae Samples
[0104]A chorionic villae sample (about 10 mg) was sonicated twice by 20 seconds in 50 μl of cold distilled water (Heat Systems-Ultrasonics, Inc., model W225R).
[0105]After removing 10 μl for protein determination, the sonicated cells were spotted on filter paper (Schleicher and Schuell N° 903, Keene, N.H., USA) and allowed to dry for 6 hours at room temperature (22° C.) on a flat non-absorbing surface. See Lowry et al., J. Biol. (hem. 193:265-275 (1951). The spotted filter paper was stored on plastic bags at −20° C. until analysis.
[0106]A sample for analysis was prepared by punching out a 3mm-diameter circle with a standard paper punch from the dried chorionic villae spots on the filter paper.
example 3
Preparation of Dried Cultured Amniocytes Samples
[0107]Cultured amniocytes were suspended in 500 μl of cold phosphate saline buffer (pH 7.4). After centrifugation at 1,200 g for 5 minutes at 4° C. the supernatant was removed by aspiration. The cell pellet was resuspended in 40 μl of cold distilled water and sonicated (Heat Systems-Ultrasonics, Inc., model W225R).
[0108]After removing 10 μl for protein determination, the sonicated cells were spotted on filter paper (Schleicher and Schuell N° 903, Keene, N.H., USA) and allowed to dry for 6 hours at room temperature (22° C.) on a flat non-absorbing surface. See Lowry et al., supra. The spotted filter paper was stored on plastic bags at −20° C. until analysis.
[0109]A sample for analysis was prepared by punching out a 3 mm-diameter circle with a standard paper punch from the dried amniocytes spots on the filter paper.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com