Kit for removing bacterial endotoxin in biological product, method thereof, and preparation method of biological product
A technology of bacterial endotoxin and biological products, which is applied in the preparation method of peptides, biochemical equipment and methods, and the determination/inspection of microorganisms, etc. It can solve problems such as inability to separate, loss of active substances in plasmids, difficult industrial operation and control, etc. , to achieve the effect of low cost, less active material loss and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0022] Experimental example 1, preparation work before removing endotoxin from biological product samples
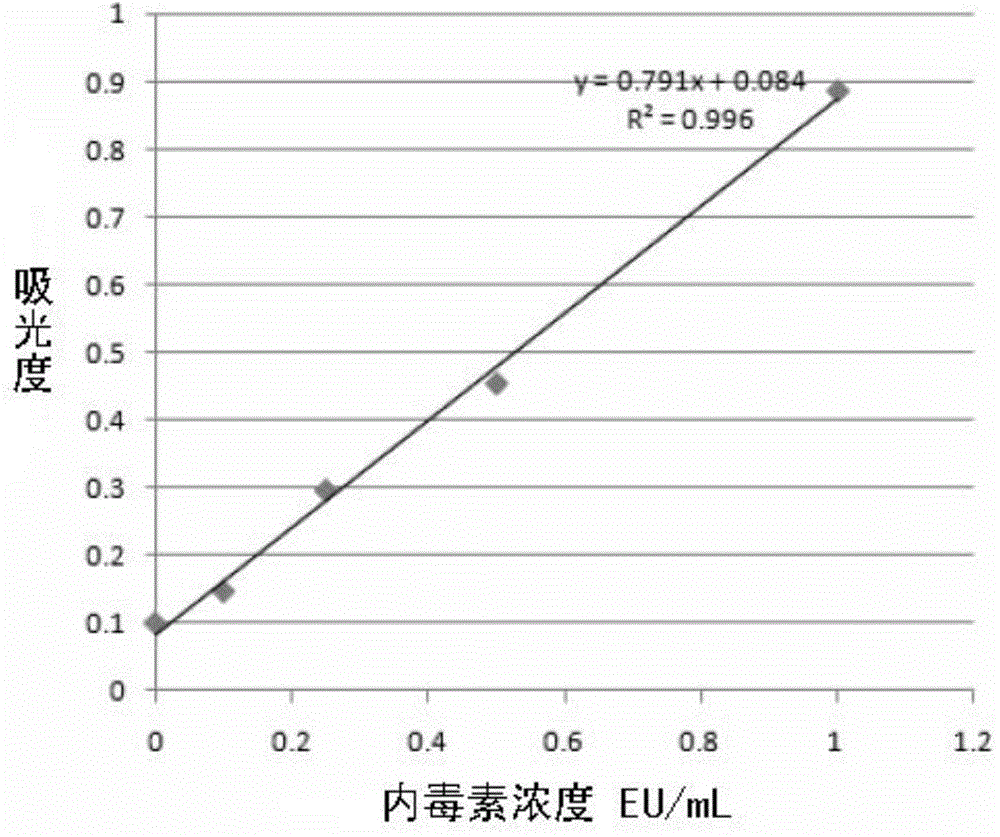
[0023] 1. Drawing of endotoxin standard substance content-absorbance standard curve
[0024] 1. Experimental materials
[0025] The chromogenic substrate Limulus kit was purchased from Xiamen Limulus Reagent Experimental Factory Co., Ltd.; the experimental equipment used was free of endotoxin.
[0026] 2. Preparation of standard endotoxin solution and drawing of standard curve
[0027] All were carried out in accordance with the instruction manual of the chromogenic substrate LAL kit provided by Xiamen Limulus Reagent Experimental Factory Co., Ltd.
[0028] Measure the absorbance of the endotoxin standard substance by photometry (see Table 1), and take the absorbance as the Y axis and the endotoxin concentration as the X axis to draw the endotoxin concentration-absorbance standard curve (see Table 1). figure 1 ), obtain the standard curve equation of y=0.791X+0.084 (R...
Embodiment 1
[0046] Example 1. Removal of endotoxin in samples of endotoxin-removing biological products
[0047] 1) Take the endotoxin-removing biological product sample obtained by the method in the third part of Experimental Example 1, and add an anionic surfactant to it until the final concentration of the anionic surfactant is not less than 1 wt% (the concentration of the anionic surfactant depends on the endotoxin Concentration, the higher the concentration of endotoxin, the concentration of anionic surfactant also needs to increase correspondingly, generally around 5-10wt%), after mixing evenly, let it stand for 5min; the anionic surfactant can be sodium dodecyl sulfate (SDS), Sodium deoxycholate (SDC), sodium secondary alkyl sulfate, sodium fatty alcohol polyoxyethylene ether sulfate, sodium oleyl alcohol sulfate, sodium N-oleoyl polypeptide, sodium alkylbenzenesulfonate, α-olefin sulfonic acid Sodium, sodium alkyl sulfonate, alpha-sulfomonocarboxylate, fatty acid sulfoalkyl ester,...
experiment example 2
[0051] Experimental example 2, endotoxin detection interference test
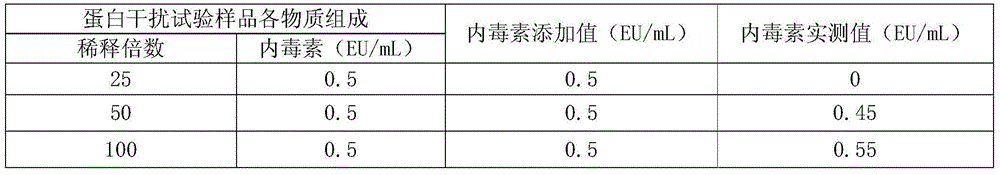
[0052] In order to exclude the influence of the absorbance of biological products on the absorbance of endotoxin, an interference test was carried out to screen out the concentration of biological products with the least degree of interference to the detection of endotoxin. The operation process of the interference test was carried out in accordance with the "interference test of the test product" in the instruction manual of the chromogenic substrate LAL kit provided by Xiamen Limulus Reagent Experimental Factory Co., Ltd.
[0053] 1. Interference test of protein sample solution on endotoxin detection
[0054] Since the protein sample solution is added with a certain concentration of other substances when the endotoxin is removed, it needs to be diluted to a certain multiple before determination to eliminate interference.
[0055] The endotoxin-removing protein sample solution (pH7-8) obtained in step 3) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com