Cell free biosynthesis of high-quality nucleic acid and uses thereof
A high-quality, cell-free technology that can be used in fermentation and other directions to solve problems such as being unsuitable for cell-free nucleic acid production, and achieve the effects of effective absorption, simplified purification steps, and increased fidelity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1 - Synthesis and cell-free amplification of β-galactosidase (LacZ-DU)
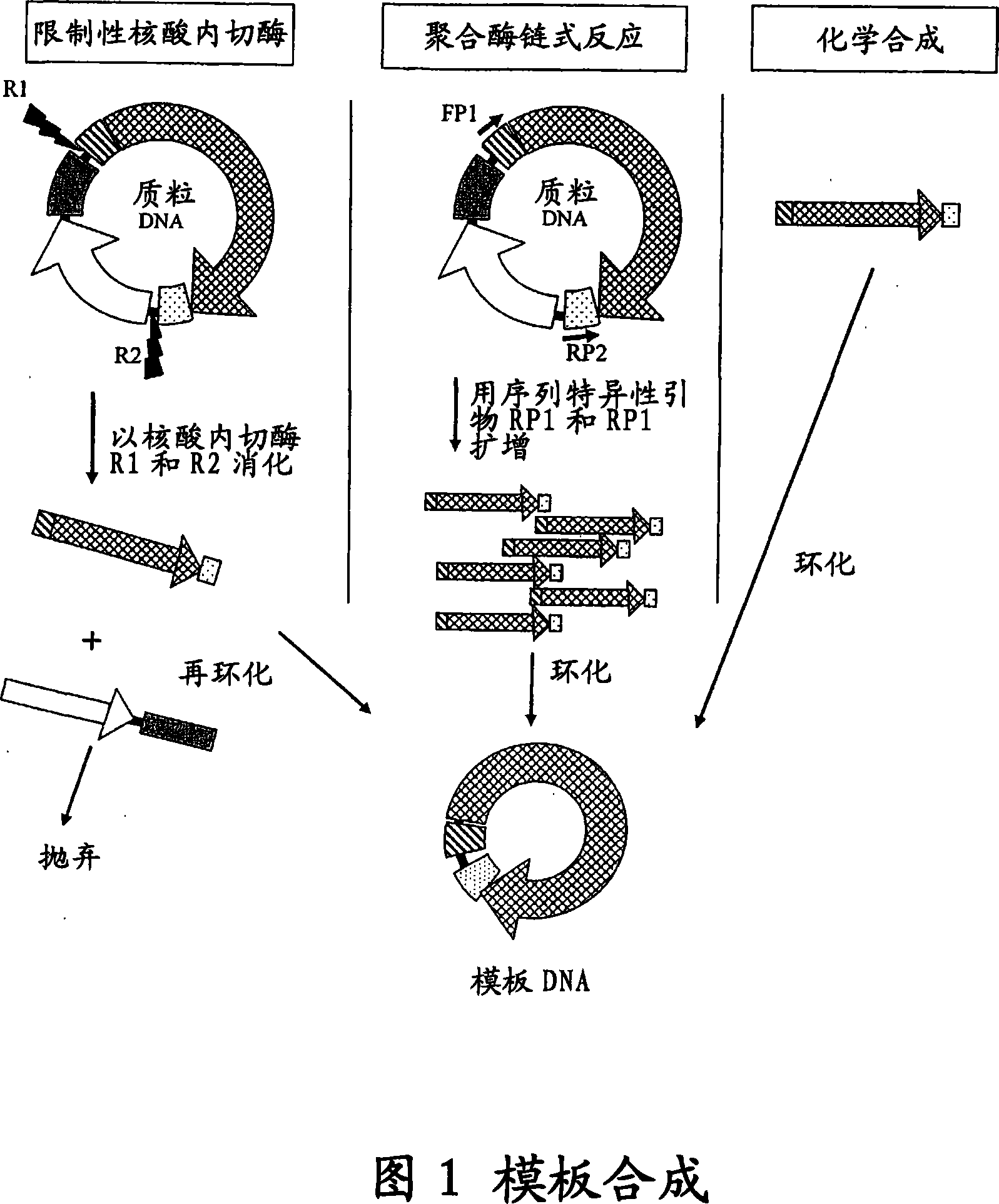
[0094] a) Plasmid-based template pSV-β-galactosidase vector (Promega, Madison, WI, USA) was partially digested with EcoR I and Pst I. An approximately 4.2 kb fragment (LacZ-DU) containing the CMV promoter, Lac Z ORF and SV40 small T antigen termination sequence was isolated, blunt-ended with T4 DNA polymerase and cloned into pGEM TM -Sma I site of 7Zf(+) (Promega, Madison, WI, USA), resulting in pGEM-LacZ-DU vector. LacZ-DU was then excised from pGEM-LacZ-DU with Xba I, gel purified and circularized with T4 DNA ligase (New England Biolabs, Beverly, MA, USA) following the manufacturer's recommendations.
[0095] b) PCR-based template using a forward primer (5'-CG GGATCC GACTCTTCGCGATGTAC-3'), reverse primer (5'-CG GGATCC CAGCATGCCTGC-3'), by pVAX TM The 200-GW / lacZ vector (Invitrogen Carlsbad, CA, USA) amplifies LacZ-DU. Containing 200ng of each primer, 10ngpVAX TM 200-GW / lacZ vector...
Embodiment 2
[0103] Example 2 - Synthesis and cell-free expansion of luciferase (Luc-DU)
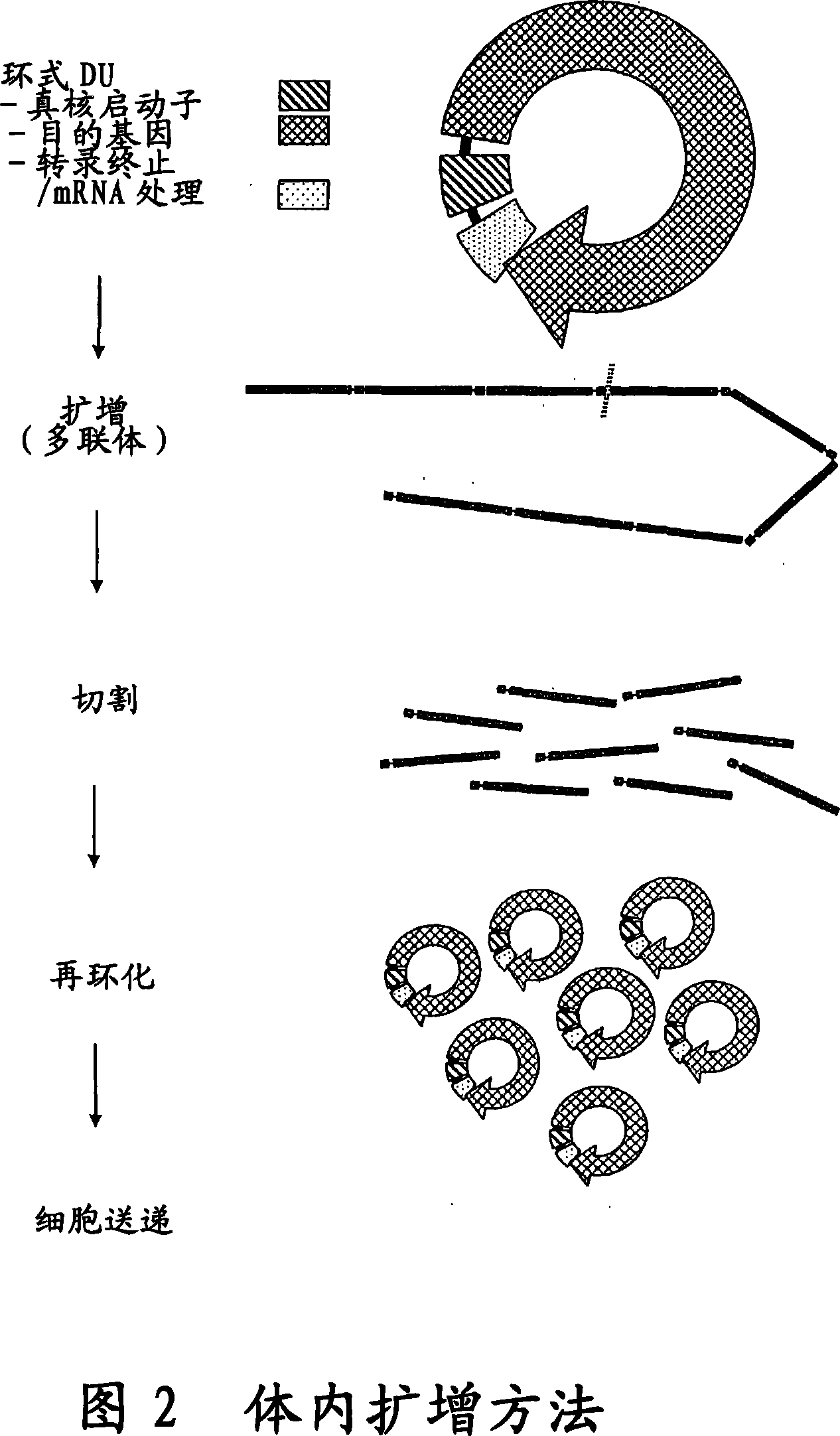
[0104] The pGL3 vector (Promega Corp. Madison, WI, USA) was digested with Sal I and Xho I. A 2.17 kb fragment containing the SV40 promoter, luciferase ORF, and SV40 small T antigen termination sequence (Luc-DU) was isolated, purified, and treated with T4 DNA ligase (Invitrogen, Carlsbad, CA, USA) following the enzyme manufacturer's recommendations. ) re-circularized (re-circularized).
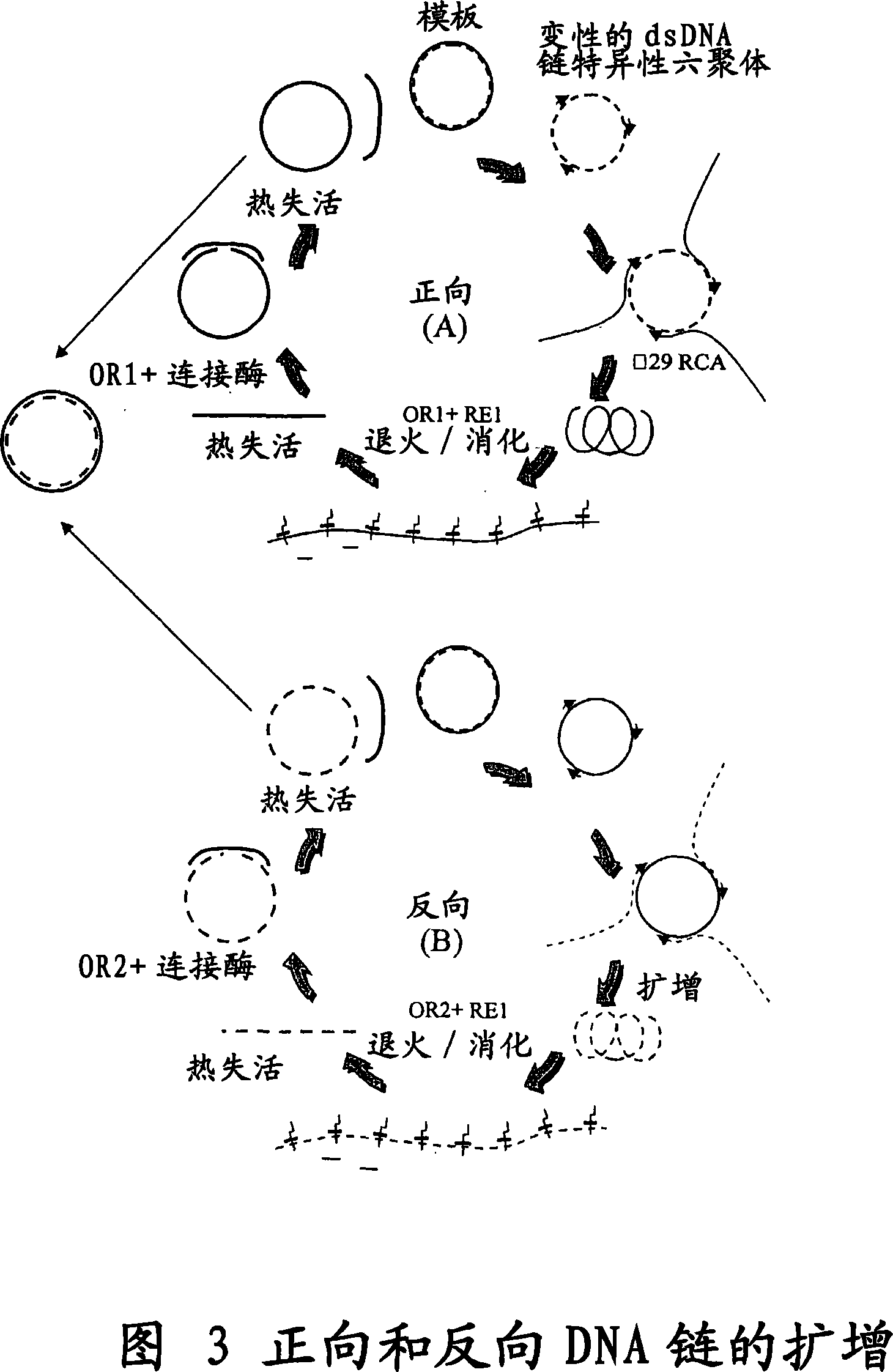
[0105] a) Cell-free amplification will contain six base primers (hexamers) 5'-ApApTpTp with a concentration of 400pmol s Gp s C-3' and 5'-ApGpCpAp s AP s T-3' and 10ng / 25μl cyclic Luc-DU reaction in 40mM Tris-HCl pH8, 10mM MgCl 2 Heat to 95°C for 3 minutes and cool to room temperature. Add Phi29 DNA polymerase (10U, NewEngland Biolabs, Beverly, MA, USA), 1mM dNTPs (25 / 25 / 25 / 25), 5% glycerol, 0.7U yeast inorganic pyrophosphatase (Sigma, St.Louis, MO, USA) and 100 μg / ml BSA. At 30°C, in 50mM Tris-HCl pH7.5, 10mM...
Embodiment 3
[0106] Example 3 - Expression of amplified DNA in human cells
[0107] at 37°C and 5% CO 2 Human lung cancer cell A549 (ATCC) was cultivated in Dulbecco's modified Eagle medium (DMEM; Invitrogen Carlsbad, CA, USA) under environmental conditions, which supplemented 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT, USA). ), 100 U / ml penicillin, 100 μg / ml streptomycin (Invitrogen Carlsbad, CA, USA).
[0108] a) DNA transfection The day before transfection, A549 cells were treated with 1×10 5 Densities of cells / ml were seeded in 6-well plates. GenePORTER2 transfection reagent (Gene Therapy System, San Diego, CA, USA) was used for cell transfection according to the manufacturer's instructions. Shortly thereafter, cell-free amplified DU or parental plasmid DNA (Promega Corp. Madison, WI, USA) was mixed with 2 μg vector pssXE DNA (Chen and McMicken, Gene Ther 10:1776-1780, 2003) in 50 μl of DNA dilution B. ) were mixed and incubated at room temperature for 5 min. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com