Separation and purification of recombinant human blood vessel endothelia cell growth factor, chemical labeling in vitro and use thereof
A technology of biotin labeling and cells, which is applied in the field of recombinant VEGF protein and biology, can solve the problems of no separation and purification of recombinant VEGF protein method or data, cumbersome process, and limitations of industrial scale production and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1: Construction of VEGF165 expression vector.
[0085] 1.1 PCR amplification of VEGF165 gene
[0086] Forward upstream primer primer (VEGF-F-EcoR I): ggg tag aat tc g cac cca tgg cag aag gag ga
[0087] Reverse Downstream Primer Primer (v165-R-Not I): gga ttc gcg gcc gcc c gc ctc ggc ttg tca ca
[0088] PCR amplification system:
[0089]
[0090] PCR amplification conditions: 94°C for 5 minutes / 94°C for 30 seconds, 55°C for 30 seconds, 72°C for 40 seconds, a total of 20 cycles.
[0091] After PCR amplification of VEGF165 gene was completed, a small amount was taken and analyzed by agarose gel electrophoresis. There was an amplified band at about 500 bp, and the size of the fragment was in line with the expectation.
[0092] 1.2 Construction and identification of VEGF165-pPic9K plasmid
[0093] The VEGF165 gene PCR amplified fragment and the pPic9K plasmid were digested with EcoR I and Not I respectively, and then separated and recovered by agarose gel...
Embodiment 2
[0094] Example 2: Expression Vector Transformation of GS115 Host Bacteria
[0095] 2.1 The VEGF165-pPic9K plasmid was prepared in 100ml bacterial liquid by alkaline lysis-PEG purification method. For specific steps, refer to the relevant chapters of "Molecular Cloning III".
[0096] 2.2 Take two tubes of about 15ug VEGF165-pPic9K plasmids and linearize them thoroughly with 30U Sal I and 30U Bgl II respectively. After digestion, the plasmid was precipitated with ethanol and redissolved with 5ul ultrapure sterile deionized water.
[0097] 2.3 Preparation of GS115 Competent Cells
[0098] (1) Inoculate a single clone in 3ml YPD medium and culture overnight at 30°C
[0099] (2) Put 300ul of the above-mentioned fresh bacterial solution in 300ml of YPD medium and culture at 30°C for 18 hours. At this time, the OD value is about 1.3.
[0100] (3) Bacteria were collected by centrifugation at 1500 g for 5 minutes, and then resuspended with 300 ml of ice-cold sterile deionized wate...
Embodiment 3
[0123] Example 3: Detection of VEGF target protein
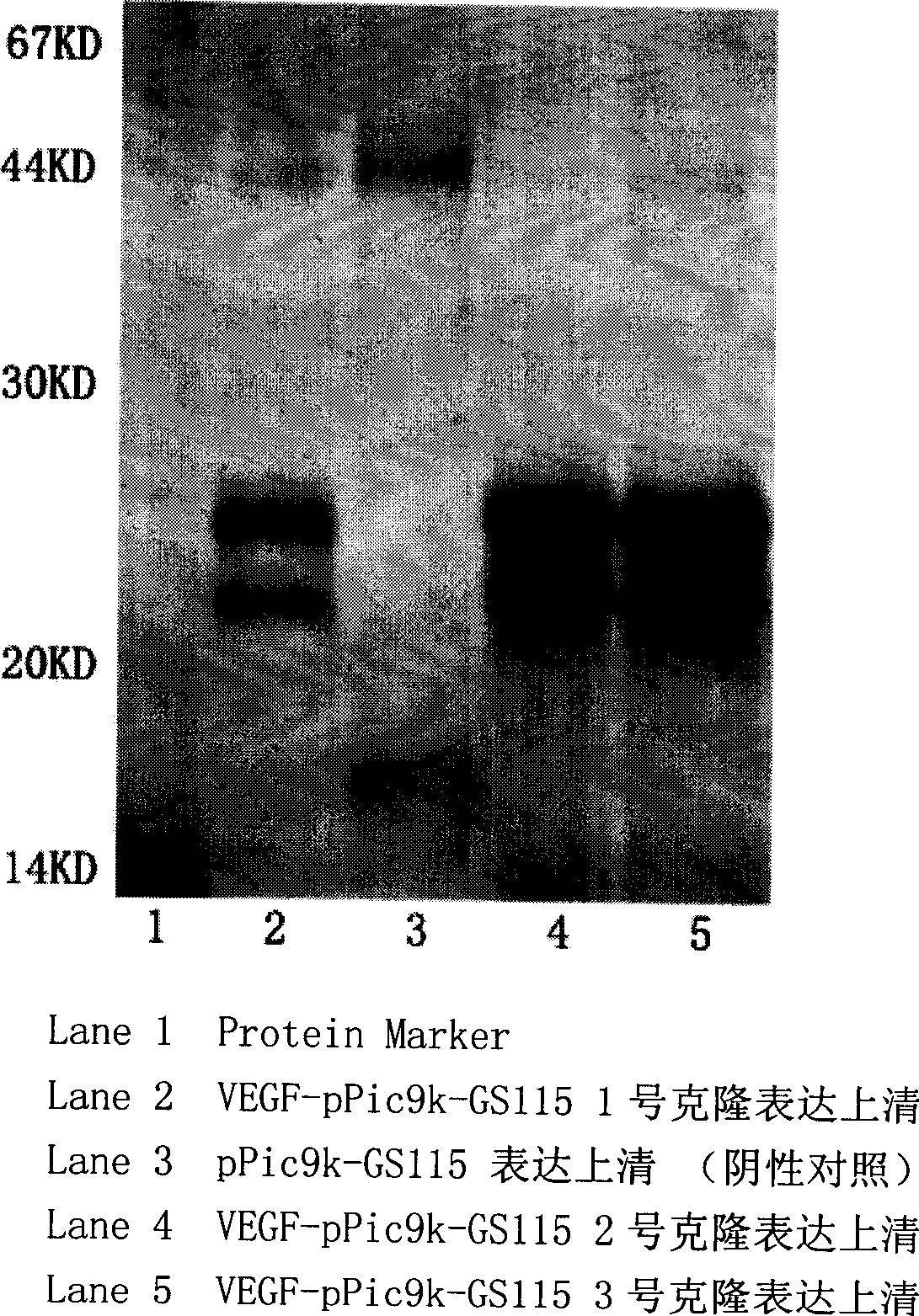
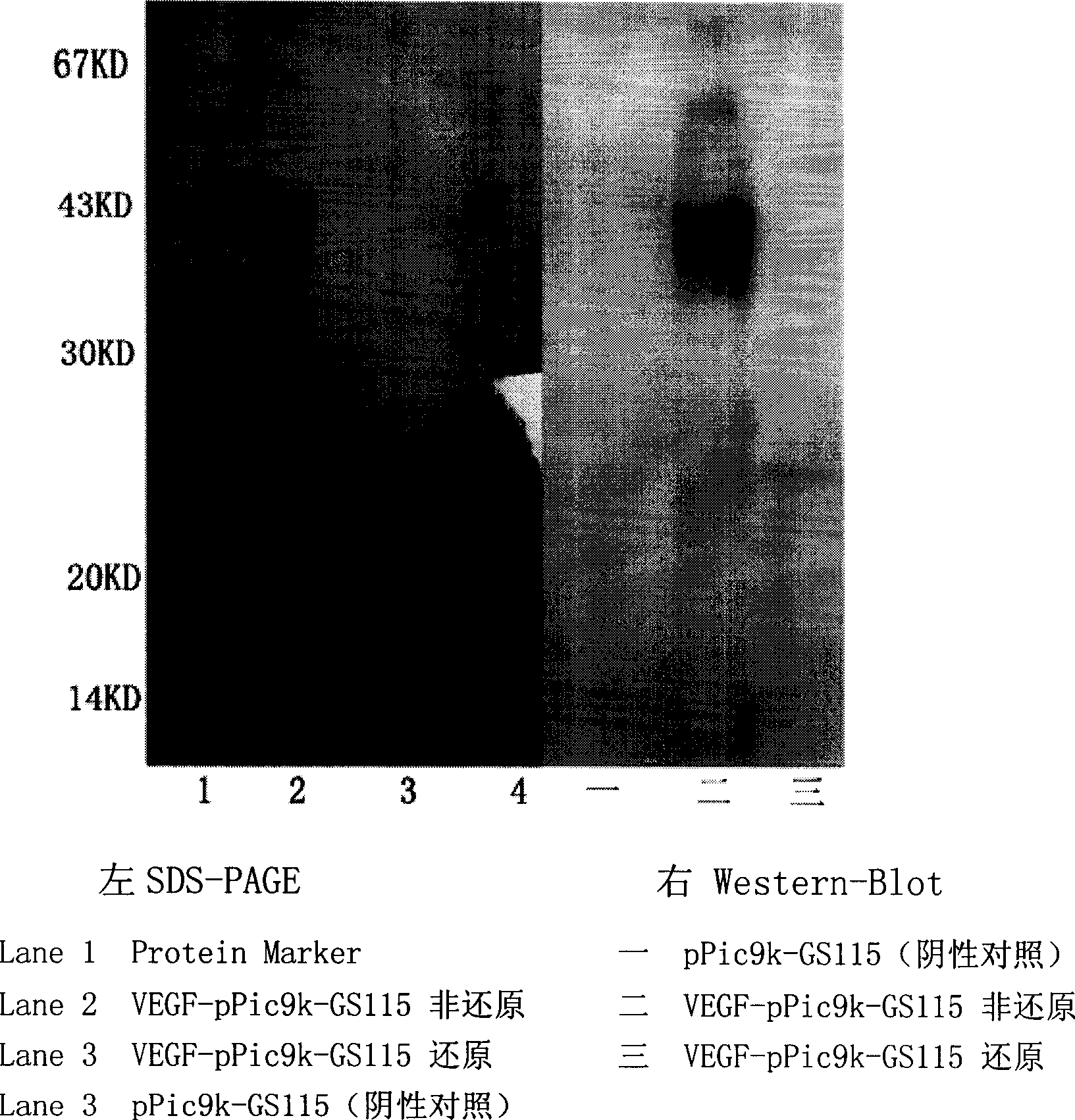
[0124] The target protein was detected by methods such as SDS-PAGE and Wester-blot. The correct expression plasmid identified by DNA sequencing was transformed into GS115 yeast host bacteria by electroshock transformation method, and thousands of recombinant clones were obtained. Subsequently, screening was performed with gradient concentrations of G418, and dozens of colonies that grew well on YPD plates containing 4 mg / ml G418 were obtained. Three clones were picked for induced expression. After 96 hours of induction, the expression supernatant was collected by centrifugation for SDS-PAGE and Western-blot analysis, and the expression supernatant of the plasmid pPic9K transformed clone was used as a negative control. The result is as figure 1 : Compared with the negative group (lane3), after VEGF165-pPic9K-GS115 expression supernatant was reduced by DTT, specific bands (lane2, 4 and 5) can be seen at about 22-25KD; the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com