Scorpion peptide for treating arrhythmia and its preparing process and application
A technology of antiarrhythmic peptides and active peptides, which is applied to high-efficiency biologically active scorpion antiarrhythmic peptides, produces scorpion antiarrhythmic peptides, and prepares antiarrhythmic drugs. It can solve the problem of limited application and anti-nerve excitatory effects It can achieve anti-arrhythmia effect, prolong the duration of epileptic seizures, and obviously anti-arrhythmia effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0032] The recombinant scorpion antiarrhythmic peptide was hydrolyzed according to the conventional method, and the components of the peptide were analyzed with a 121-MB Beckman amino acid analyzer according to the method for analyzing amino acids. The hydrolysis process: Reagent A: in vacuum, 6N HCl, 110 ° C for 24 hours. Reagent B: 4N methanesulfonic acid, 0.2% 3-(2-aminoacetic acid) indole, 110° C., in vacuum for 24, 48, 72 hours. Dissolve the sample in 1.5ml of a solution containing 40% n-propanol and 0.1% trifluoroacetic acid, fill each tube with 0.1ml, blow dry with dry nitrogen, add reagents A and B, seal the container in vacuum, and collect the hydrolysis contents. The results are as follows: amino acid mole % aspartic acid (Asp+Asn) 11.4 threonine 5.0 serine 6.5 glutamic acid (Glu+Gln) 6.6 proline 1.8 glycine 17.8 alanine 3.51 / 2 cysteine 13.1 Valine 0.0 Methionine 0.0 Isoleucine 3.4 Tyramino 6.5 Phenylalanine 1.6 Lysine 7.8 Histidine 0.0 Tryptophan 8.3 Arginine 1.8...
Embodiment 3
[0033] The samples were decomposed by Edman using Applied Biosystems 476A sequencer, and the obtained PTH amino acids were analyzed by Applied Biosystems Model120APTH-analyzer. The results showed that the sequence of ten amino acids at the N-terminal was "DGYIRGSNGC". Example 4: Circular Dichroism Determination of Recombinant Scorpion Antiarrhythmic Peptide
Embodiment 4
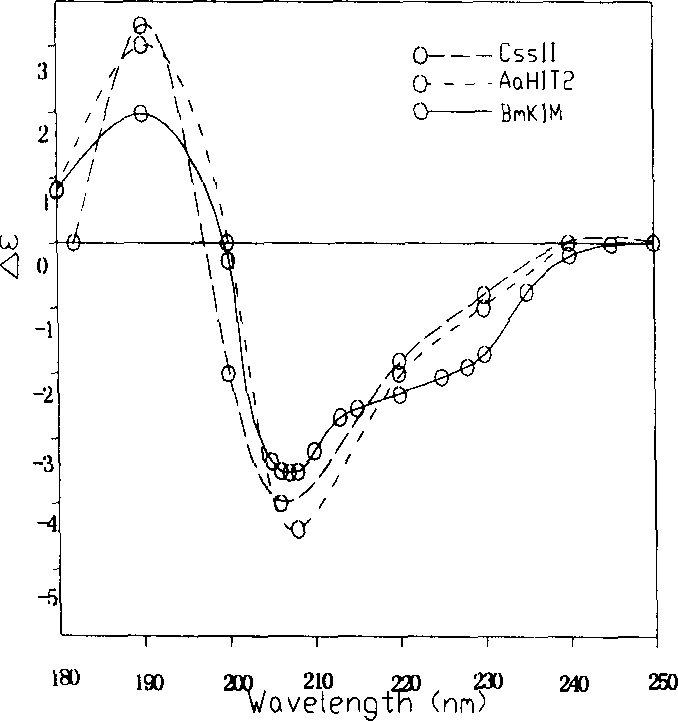
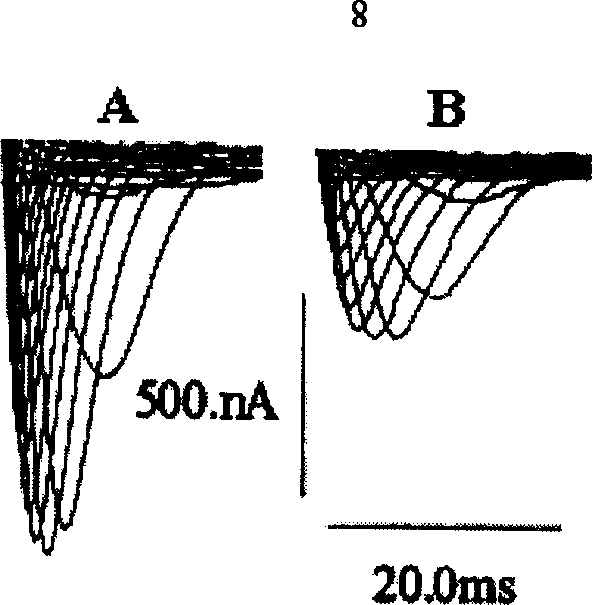
[0034] Take a sample of 0.1-0.3mg / ml and put it into a 2mm quartz sample tank, and use a Jasco-715 chromatograph to perform circular dichroism measurement in the wavelength range of 250-180nm at 25°C. attached figure 2 It shows that the secondary structure of the recombinant scorpion antiarrhythmic peptide is similar to that of other scorpion venom polypeptides. Example 5: Activity detection of recombinant scorpion anti-arrhythmic peptide
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com