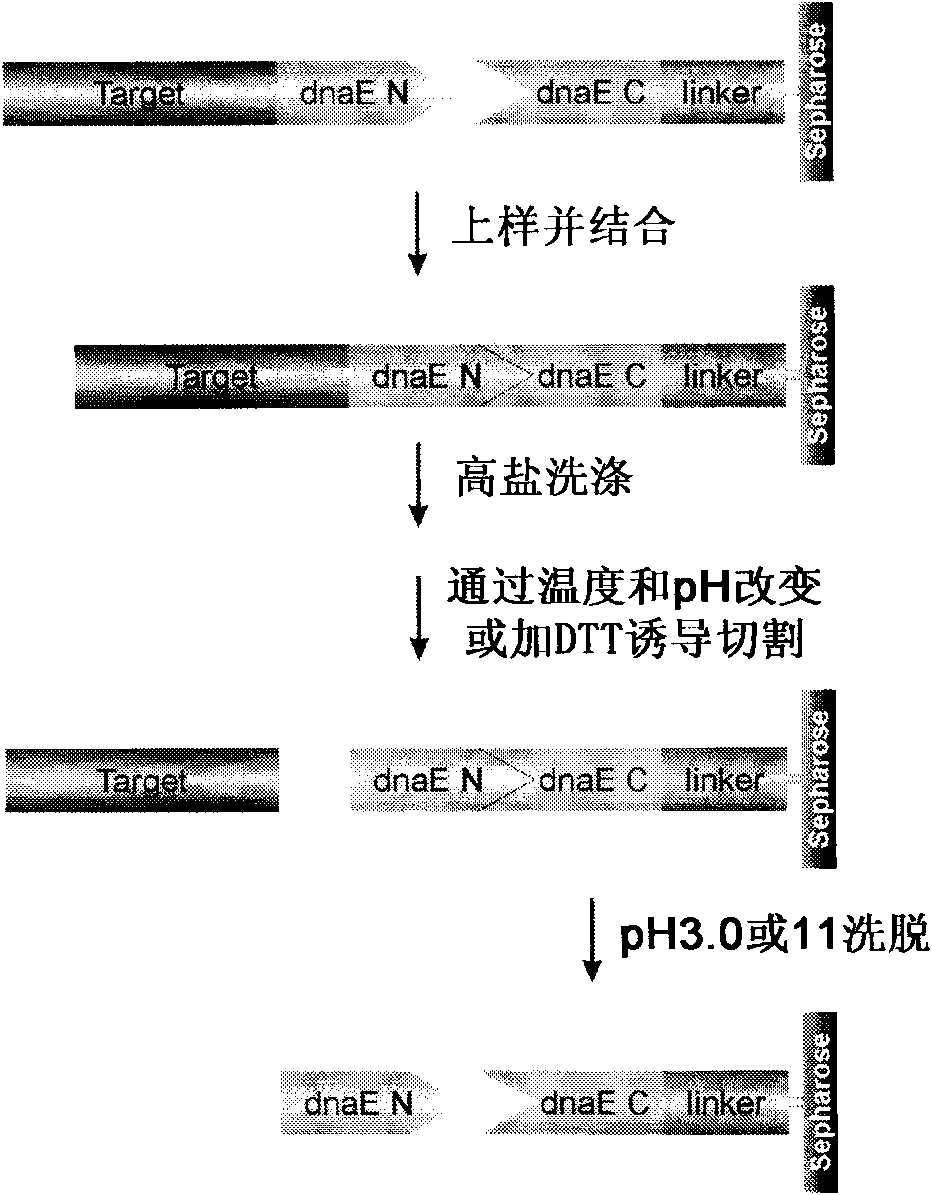
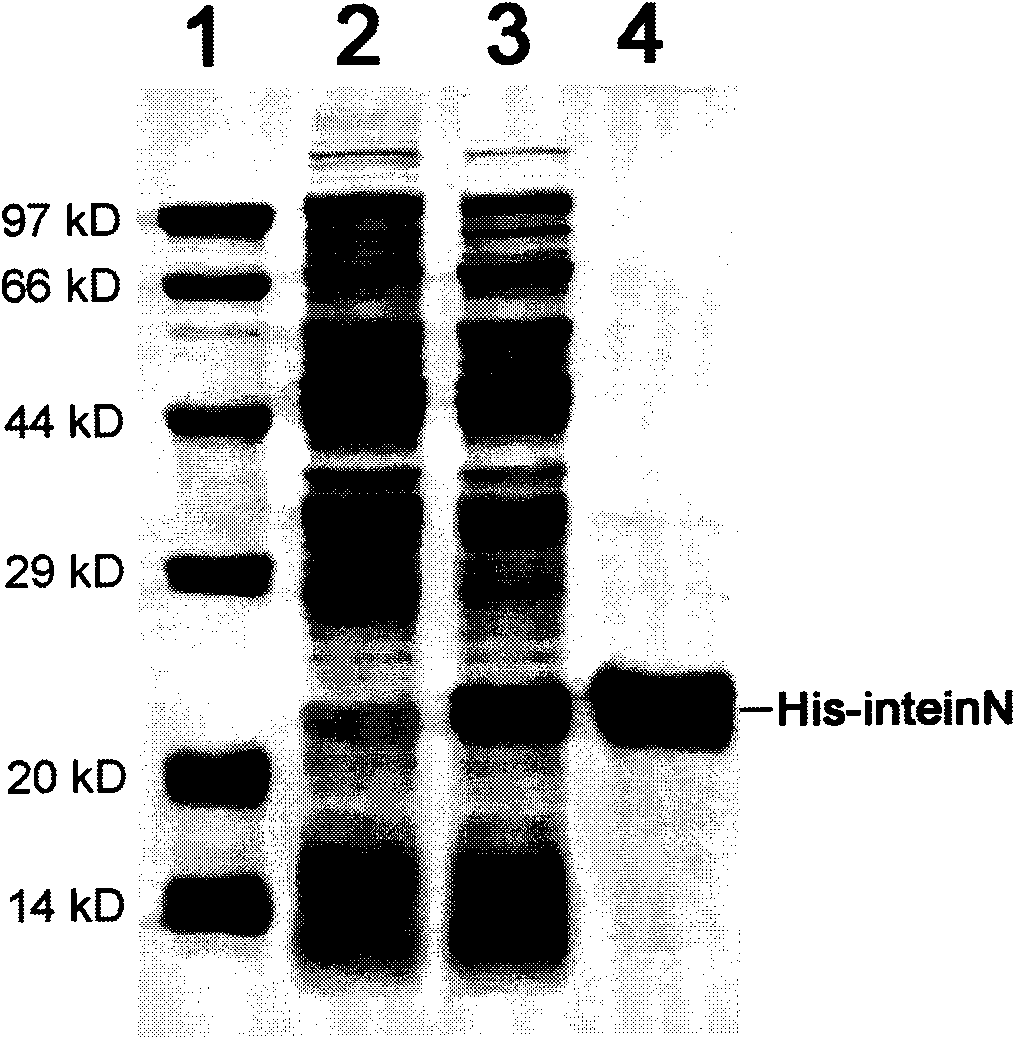
Preparation of recombinant polypeptide by intein with trans-splicing function
A protein intron and trans-splicing technology, which is applied in the field of recombinant polypeptide preparation, can solve the problems such as the inability of protein introns to ligand and reduce the proportion of target polypeptide, and achieve the effects of simple structure, increased proportion and increased yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0057] Preparation of recombinant Erythrina trypsin inhibitor (ETI):
[0058] 1. Construction of pET28a / His-inteinN and pET28a / inteinC-ETI expression plasmids
[0059] Construction of pET28a / His-inteinN expression plasmid: Using pTwin1 plasmid as a template, the gene sequence (inteinN) encoding the N-terminal splicing domain of the Ssp DnaB intein was amplified by PCR. PCR amplification conditions were: 5.0 fmol of pTwinl plasmid, 50 pmol of upstream and downstream primers, 0.25 U pyrobest enzyme, denaturation at 94°C for 2 minutes, followed by 26 cycles: 94°C, 30s; 55°C, 1min; 72°C, 60s, After purified by agarose electrophoresis, the PCR product was recovered with a DNA fragment recovery kit, digested with NdeI / XhoI double enzymes, recovered a large fragment (about 550bp), mixed with the expression plasmid pET28a digested by NdeI / XhoI, added T4 DNA ligase, and used The ligation product was transformed into E.coli DH5α competent cells, positive clones were identified by NdeI / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com