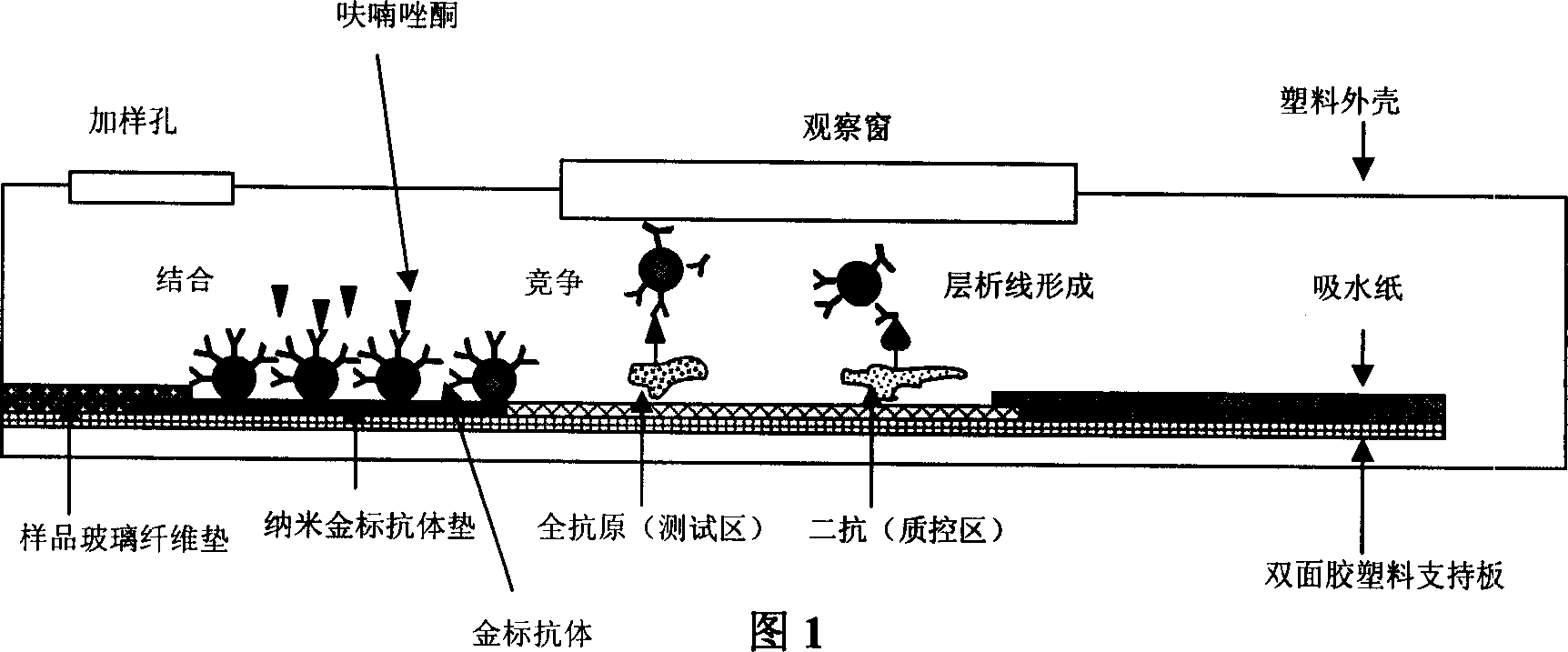
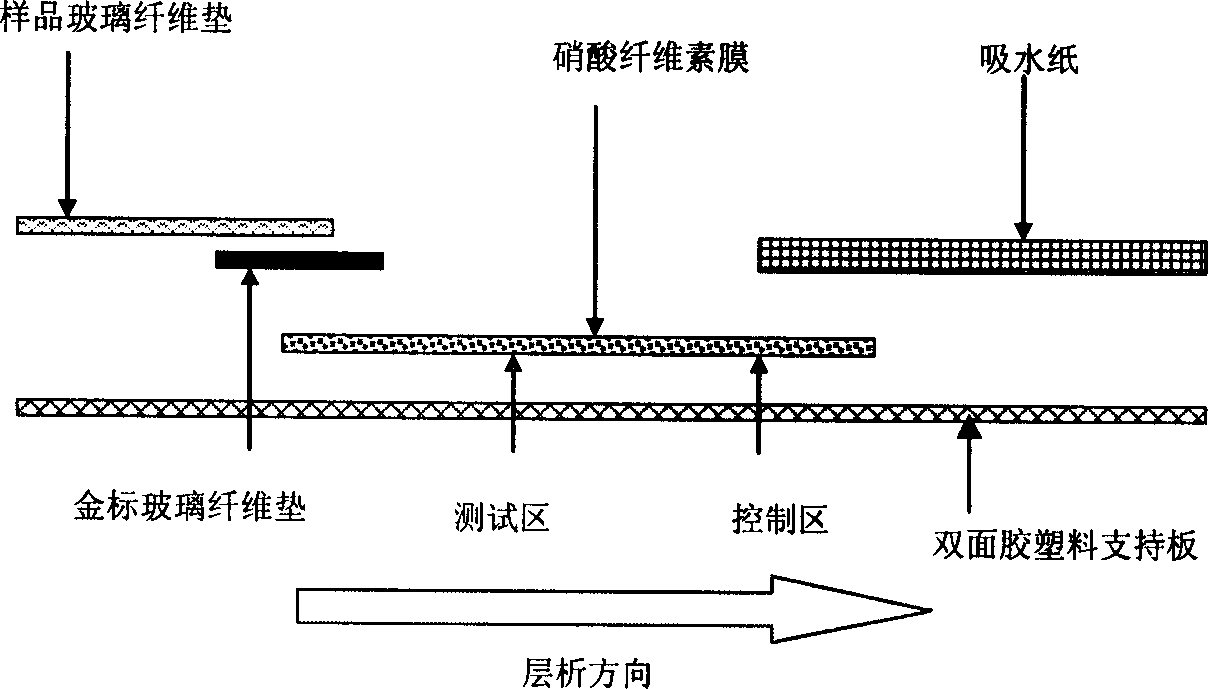
Preparation method of immune nanometer gold test paper strip for fast detecting furazolidone
A furazolidone and nano-gold technology is applied in the field of preparation of immune nano-gold test strips, which can solve problems such as time-consuming and manpower, and achieve the effects of being convenient to carry, shortening detection time, and lowering the lower detection limit.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] ① Synthesize furazolidone-bovine serum albumin conjugates and furazolidone-catalase conjugates, and identify the binding ratio of these two conjugates. Before the identification of the binding ratio, furazolidone was made into a 225 μg / ml solution, and furazolidone-bovine serum albumin conjugate and furazolidone-catalase conjugate were made into 0.4mg / ml and 0.32mg / ml aqueous solutions respectively , and at the same time prepare 0.4mg / ml bovine serum albumin solution and 0.32mg / ml catalase aqueous solution respectively, and then carry out ultraviolet scanning, according to OD 280 Calculate the respective molar absorptivity ε, measure respectively the binding ratio of furazolidone and bovine serum albumin and the binding ratio of furazolidone and catalase, binding ratio=[ε280 (conjugate)-ε(bovine serum albumin or hydrogen peroxide enzyme)] / ε280 (furazolidone). According to the calculation results, the binding ratios of furazolidone to bovine serum albumin and furazolido...
Embodiment 2
[0058]① The specific process of synthesizing furazolidone-bovine serum albumin conjugate and furazolidone-catalase conjugate and the method of identifying the binding ratio of these two conjugates are basically the same as in Example 1. The difference is that the dosage of furazolidone was reduced to 56.3 mg when synthesizing the furazolidone-catalase conjugate. The finally obtained binding ratio of furazolidone to catalase is 25:1. When this furazolidone-catalase conjugate was immobilized on the nitrocellulose membrane to carry out the immunogold nanometer test paper test experiment, under the premise of not adding the test sample furazolidone, the degree of color development was not statistically different from that in Example 1. Significant difference; but after adding the sample to be tested, furazolidone, the detection sensitivity is slightly higher than the implementation result of Example 1, but there is no statistically significant difference.
[0059] ② The specific ...
Embodiment 3
[0066] ① The specific process of synthesizing furazolidone-bovine serum albumin conjugate and furazolidone-catalase conjugate and the method of identifying the binding ratio of these two conjugates are basically the same as in Example 1. The difference is that when synthesizing the furazolidone-catalase conjugate, the dosage of furazolidone is increased to 225.2 mg. The final binding ratio of furazolidone to catalase was 82:1. When this furazolidone-catalase conjugate was immobilized on a nitrocellulose membrane to carry out the immunogold nanometer strip test experiment, under the premise that no test sample furazolidone was added, the degree of color development was significantly lighter than that of Example 1. and after adding the sample to be tested, furazolidone, the detection sensitivity also decreased significantly, indicating that the synthesized furazolidone-catalase conjugate and antibody immunoreactivity is not strong, resulting in significant detection accuracy and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com