Method for measuring cystatin c in human body fluid
A technology of cysteine protease and determination method, applied in the field of determination of cysteine protease inhibitor C in human body fluid, can solve the problem of inability to perform specific determination of cysteine protease inhibitor C, polyclonal antibody Low specificity and other issues, to achieve the effect of low cost and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0035] Hereinafter, a part of the present invention will be described in detail with reference to production examples, evaluation examples, and working examples, but the present invention is not limited to these.
[0036] 〔Example of making〕
[0037] Production of High Affinity Anti-human Cystatin C Monoclonal Antibody
[0038] 100 μg of purified human cystatin C (SCIPAC) was used for one immunization. For the first immunization, 200 μL of an emulsion prepared by mixing equal amounts of human cystatin C and Freund's complete adjuvant was used, and injected into the peritoneal cavity of BALB / c mice. In the booster immunization, 200 μL of the emulsion prepared in the same way using Freund's incomplete adjuvant was used, and in order to obtain high-affinity antibodies, long-term immunization was performed by repeating the booster immunization 6 times at 2-week intervals. The antibody titer in the blood obtained by blood collection from the fundus vein of the mouse was measured ...
example 1
[0042] Selection of anti-human cystatin C monoclonal antibodies with different recognition sites
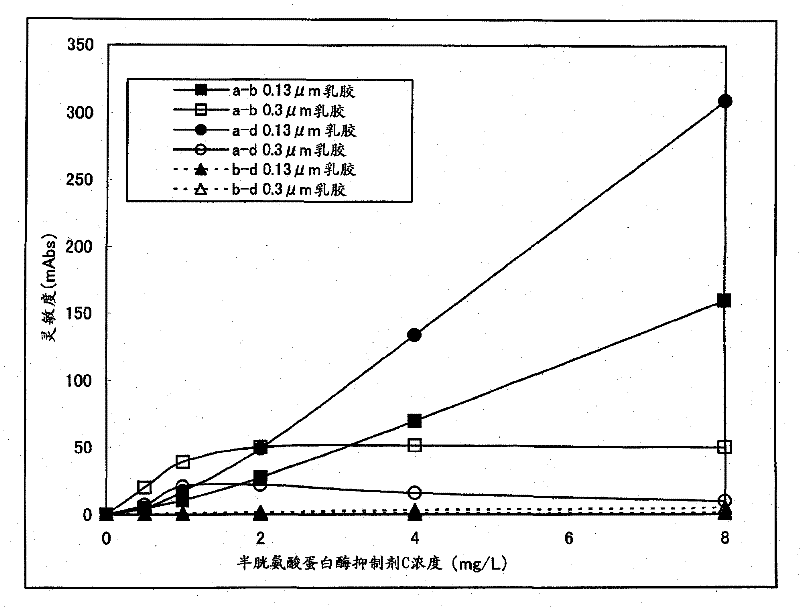
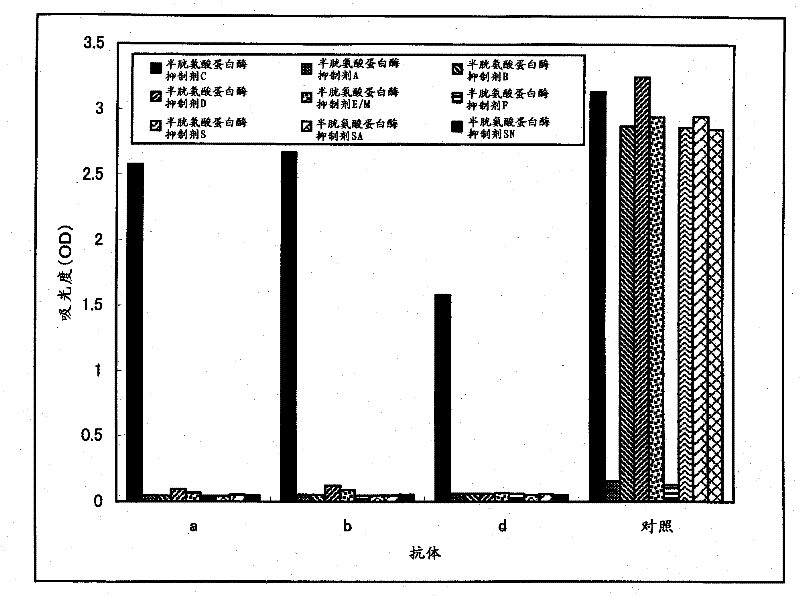
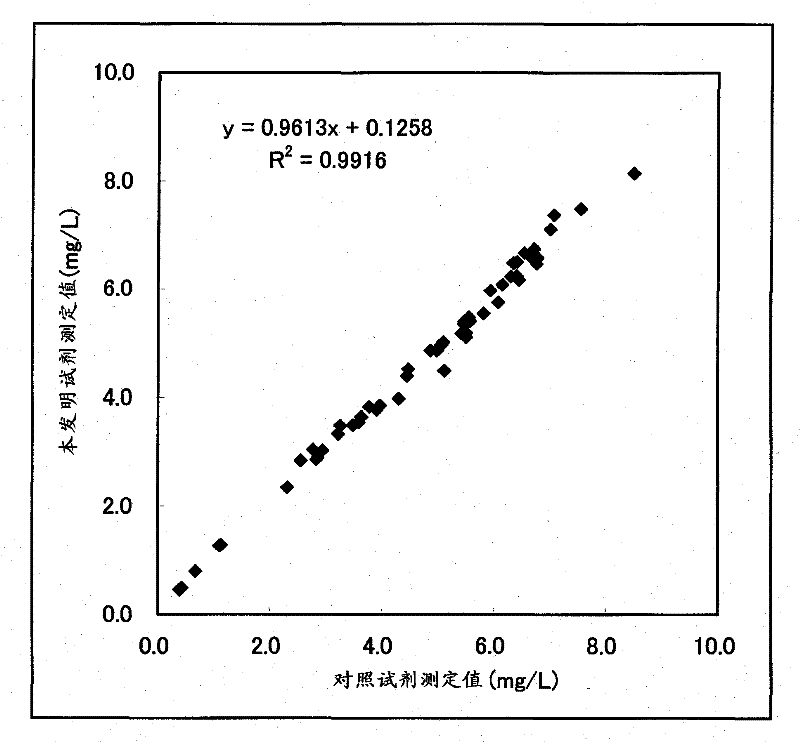
[0043] Using the reactivity in the sandwich ELISA method as an index, combinations of different recognition sites among the produced anti-human cystatin C monoclonal antibodies were searched. Specifically, 50 μL / well of PBS containing 1 μg / mL of each anti-human cystatin C monoclonal antibody was added, left to stand at room temperature for 2 hours, solid-phased on the plate, and then mixed with 0.05 Wash 3 times with 300 μL of PBS containing % Tween20, add PBS containing 0.05% Tween20 and 1% BSA at 300 μL / well, and let stand at room temperature for 1 hour. Then, after washing three times with PBS containing 0.05% Tween20, 50 μL / well of PBS containing 10 ng / mL human cystatin C was added, and the reaction was carried out at room temperature for 1 hour. After washing with PBS of % Tween20 for 3 times, add 50 μL / well of PBS containing 1 μg / mL of each biotinylated anti-human cystatin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com