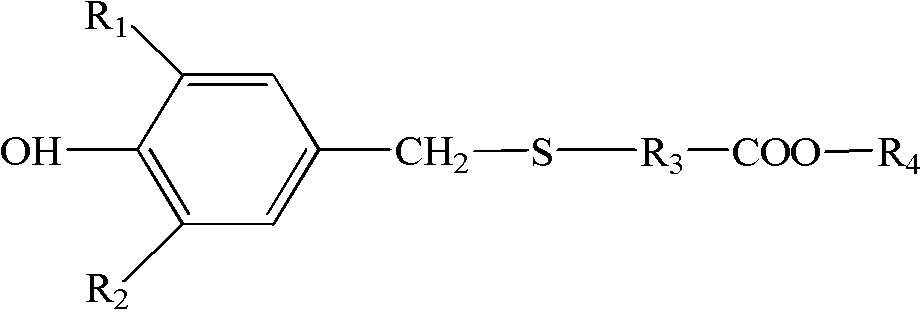
Thioether-containing hindered phenol antioxidant and preparation method thereof
A hindered phenol antioxidant and sulfide-containing technology, which is applied in the field of lubricants to achieve outstanding anti-oxidation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
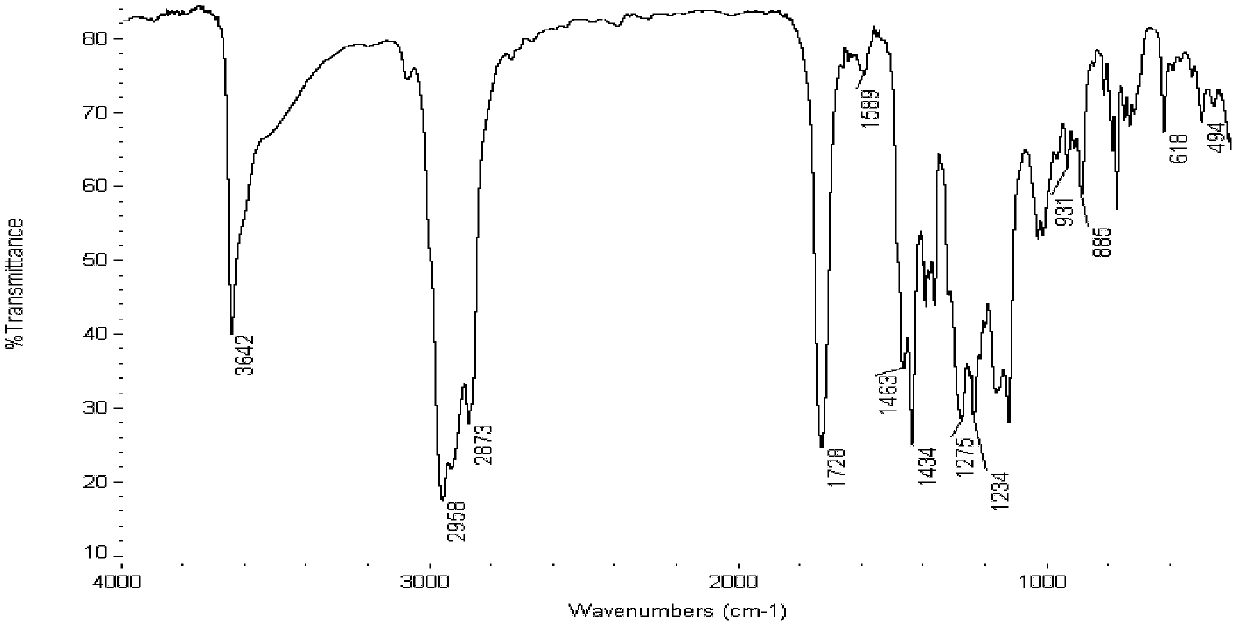
[0030] Mix 20.6g (0.1mol) of 2,6-di-tert-butylphenol, 3.6g (0.12mol) of paraformaldehyde, and 20.4g (0.1mol) of isooctyl mercaptoacetate into a 500ml four-necked bottle, and use 80mlN, N-dimethylformamide was used as a solvent, and 0.2 g (0.002 mol) of catalyst triethylamine was added under stirring conditions, gradually heated to 120 ° C, and kept for reflux reaction for 7 hours. After the reaction was completed, the product was extracted with petroleum ether, washed with water for 3 ~5 times, and then distilled under reduced pressure to obtain a yellow-brown transparent liquid product. The S content of the product is 7.31%, and the theoretical S content is 7.55%. figure 1 It is the infrared spectrogram of (3,5-di-tert-butyl-4-hydroxyl-) benzylthioglycolate isooctyl that example 1 obtains, wherein 3642cm -1 Is the absorption peak of phenol-O-H; 883cm -1 Near = C-H deformation vibration (four-substituted -C-H vibration on the benzene ring); 494cm -1 The -S-stretching vibrat...
Embodiment 2
[0033] Mix 20.6g (0.1mol) of 2,6-di-tert-butylphenol, 12.2g (0.15mol) of formaldehyde solution (37%), and 14.8g (0.1mol) of n-butyl thioglycolate into a 500ml four-necked bottle, Using 80ml of ethyl acetate as a solvent, add 0.12g (0.001mol) of catalyst N,N-dimethylaniline under stirring conditions, gradually heat to 75°C, keep reflux reaction for 4 hours, after the reaction, extract the product with petroleum ether , washed with water for 3 to 5 times, and distilled under reduced pressure to obtain a reddish-brown transparent liquid product. The S content of the product is 8.62%, and the theoretical S content is 8.74%.
Embodiment 3
[0035] Mix 12.3g (0.1mol) of 2,6-dimethylphenol, 4.2g (0.14mol) of paraformaldehyde, and 20.4g (0.1mol) of isooctyl thioglycolate into a 500ml four-necked bottle, and use 80mlN, N - Dimethylformamide as a solvent, then add 0.2 g (0.002 mol) of triethylamine as a catalyst, gradually heat to 120 ° C under stirring conditions, keep reflux reaction for 6 hours, after the reaction, extract the product with petroleum ether, wash with water 3 to 5 times, and then distilled under reduced pressure to obtain a yellow-brown transparent liquid product. The S content of the product is 9.41%, and the theoretical S content is 9.44%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Initial decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com