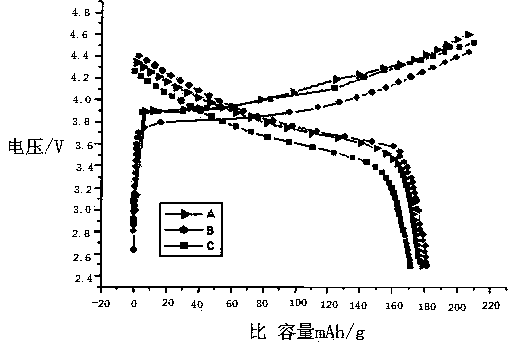
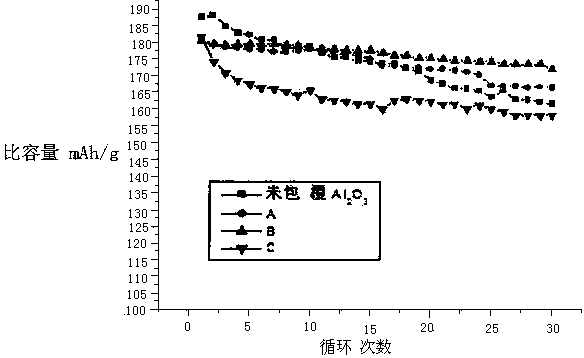
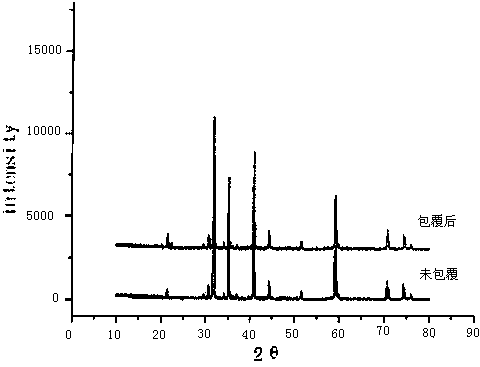
Preparation method of alumina composite nickel-cobalt lithium manganate ternary material
A technology for compounding nickel cobalt lithium manganate and ternary materials, which is applied in electrical components, electrochemical generators, battery electrodes, etc., can solve the problems of uneven dispersion and long coating cycle, and achieves improved rate performance and preparation cycle. Short, the effect of improving the transmission efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0034] The nickel salt, cobalt salt and manganese salt of this embodiment all use its acetate, the aluminum salt used for coating is aluminum nitrate, the precipitating agent is sodium hydroxide, and the complexing agent is ammonia water. Proceed as follows:
[0035] (1) According to the molar ratio of nickel, cobalt, and manganese of 1:1:1, weigh the acetate of nickel, cobalt, and manganese into a conical flask, add 80 ml of distilled water, shake to dissolve, and prepare solution A.
[0036] (2) According to the molar ratio of (Ni+Co+Mn):NaOH = 1:2.5, weigh the precipitating agent sodium hydroxide into another Erlenmeyer flask, add 50 ml of distilled water to prepare solution B.
[0037] (3) Place the three-neck round bottom flask equipped with a mechanical stirrer and a constant pressure dropping funnel in a 60°C constant temperature water bath, add 5% sodium dodecylbenzenesulfonate solution to the flask, and turn the stirrer speed to Adjust to 300 r / min, and replace the a...
Embodiment B
[0043] The nickel salt of the present embodiment, cobalt salt, manganese salt all adopt its sulfate. The aluminum source used for coating is aluminum acetate, the precipitating agent is sodium carbonate, and the complexing agent is ammonia water. Proceed as follows:
[0044] (1) According to the molar ratio of nickel, cobalt and manganese of 1.1:1.4:1, weigh the sulfate salt of nickel, cobalt and manganese into a conical flask, add 80 ml of distilled water, shake to dissolve, and prepare solution A.
[0045] (2) Press (Ni+Co+Mn): Na 2 CO 3 = 1:4 molar ratio, weigh the precipitating agent sodium carbonate in another conical flask, add 50ml of distilled water to prepare solution B.
[0046] (3) Place a three-neck round bottom flask equipped with a mechanical stirrer and a constant pressure dropping funnel in a constant temperature water bath at 60 °C, add 10% sodium dodecylbenzenesulfonate solution into the flask, and turn the stirrer speed to Adjust to 400 r / min, and replac...
Embodiment C
[0052] The nickel salt, cobalt salt, and manganese salt of the present embodiment all adopt its nitrate. The aluminum source used for coating is aluminum sulfate, the precipitation agent is ammonium bicarbonate, and the complexing agent is ammonia water. Proceed as follows:
[0053] (1) According to the molar ratio of nickel, cobalt, and manganese as 1.5:1.8:1, weigh the nitrate of nickel, cobalt, and manganese into a conical flask, add 80ml of distilled water, shake to dissolve, and prepare solution A.
[0054] (2) According to (Ni+Co+Mn): NH 4 HCO 3 = 1:6.5 molar ratio, weigh the precipitant ammonium bicarbonate in another Erlenmeyer flask, add 50 ml of distilled water to prepare solution B.
[0055] (3) Place a three-neck round bottom flask equipped with a mechanical stirrer and a constant pressure dropping funnel in a constant temperature water bath at 60 °C, add 6% sodium lauryl sulfate solution into the flask, and adjust the speed of the stirrer to 500 r / min, use nit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com