Multiple-variable dose regimen for treating TNFa-related disorders
a multi-variable, dose regimen technology, applied in immunological disorders, antibody medical ingredients, peptide/protein ingredients, etc., can solve the problems of difficult crohn's disease treatment, significant side effects, and current compounds and regimens that do not completely abate the inflammatory process, so as to improve the treatment of tnfa-related disorders.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Study of Efficacy of Multiple-Dose Therapy for Treatment of Crohn's Disease
[0468]Multiple-Variable Dose Treatment of Crohn's Disease (CD) with D2E7 (Adalimumab)
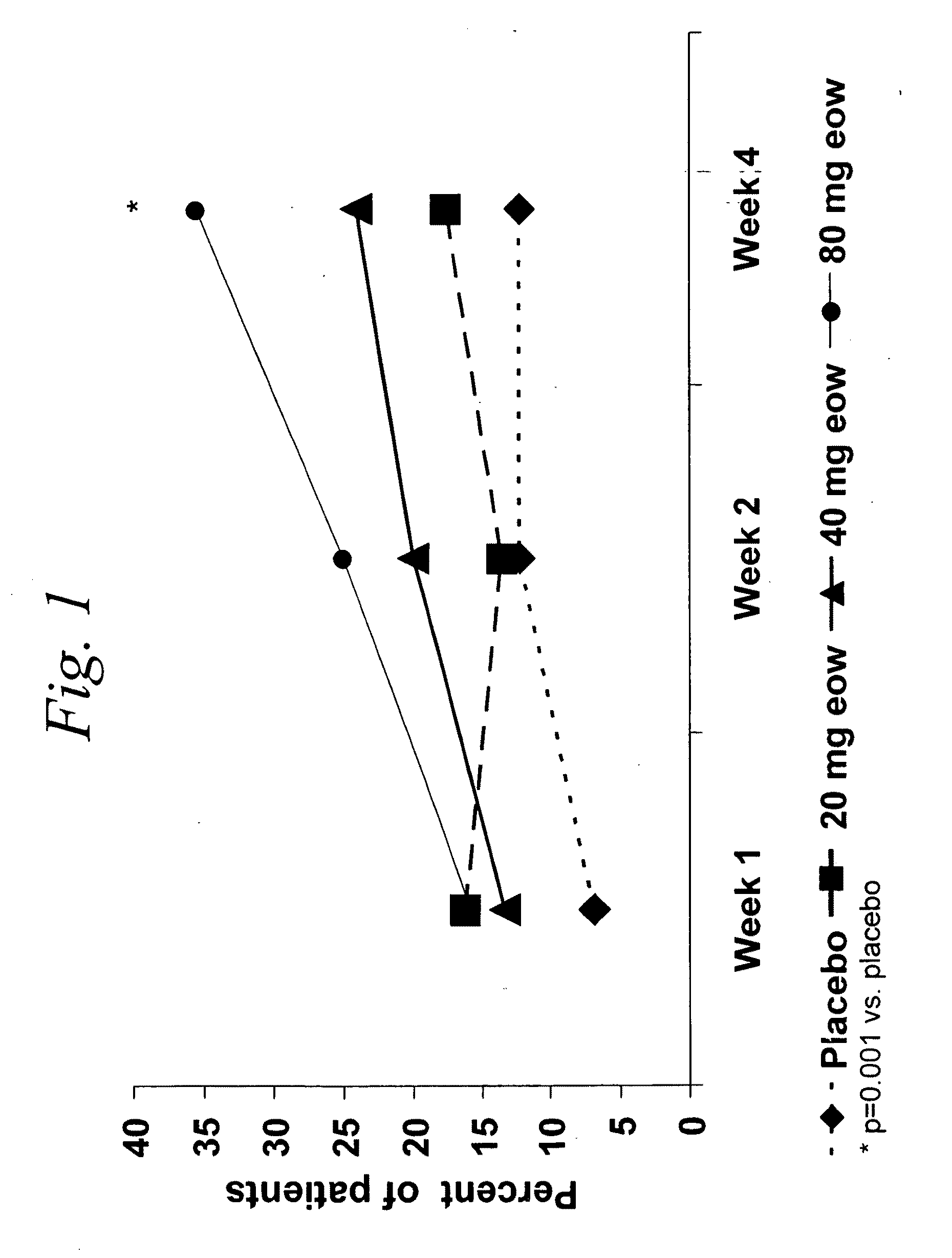
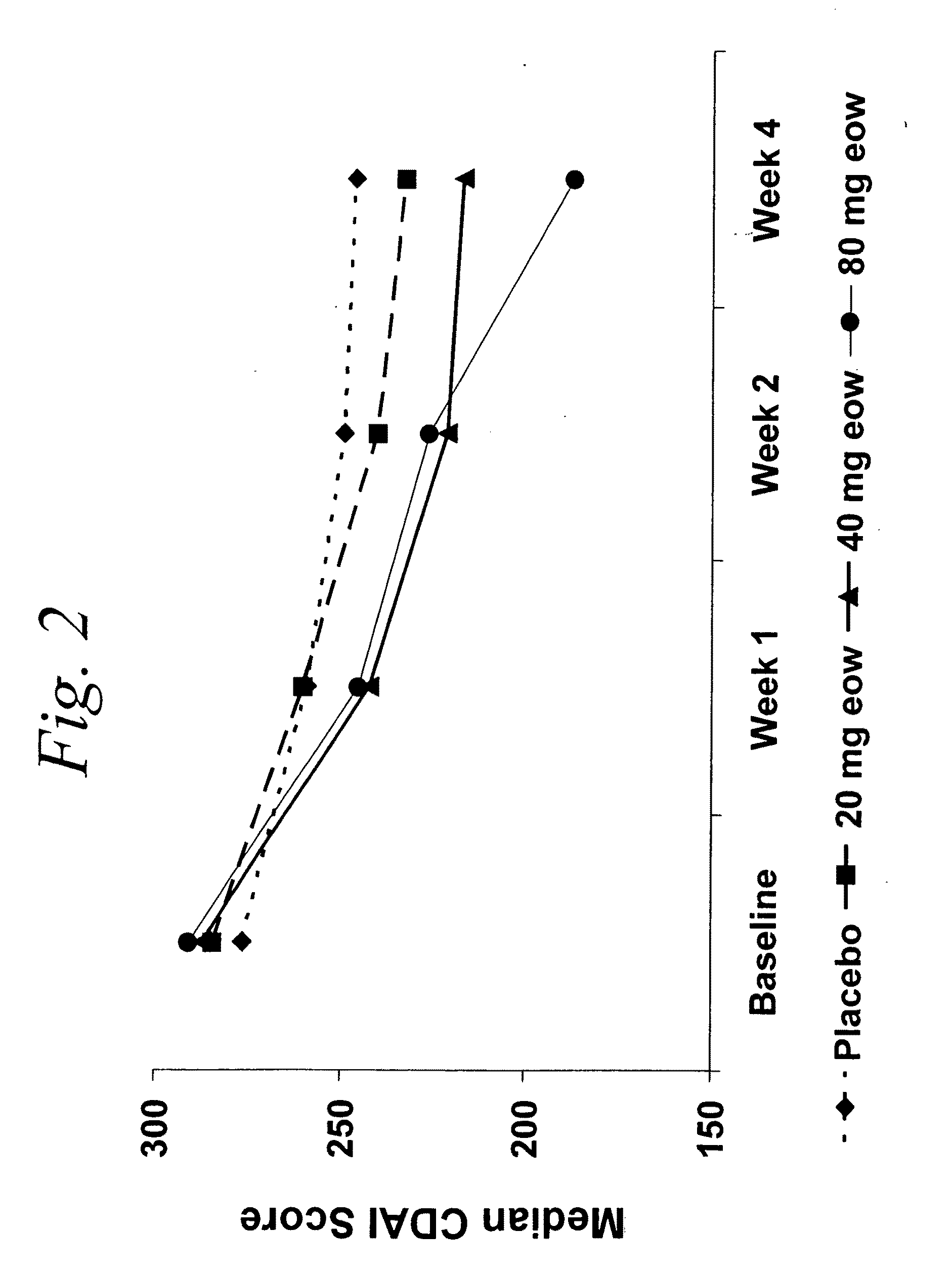
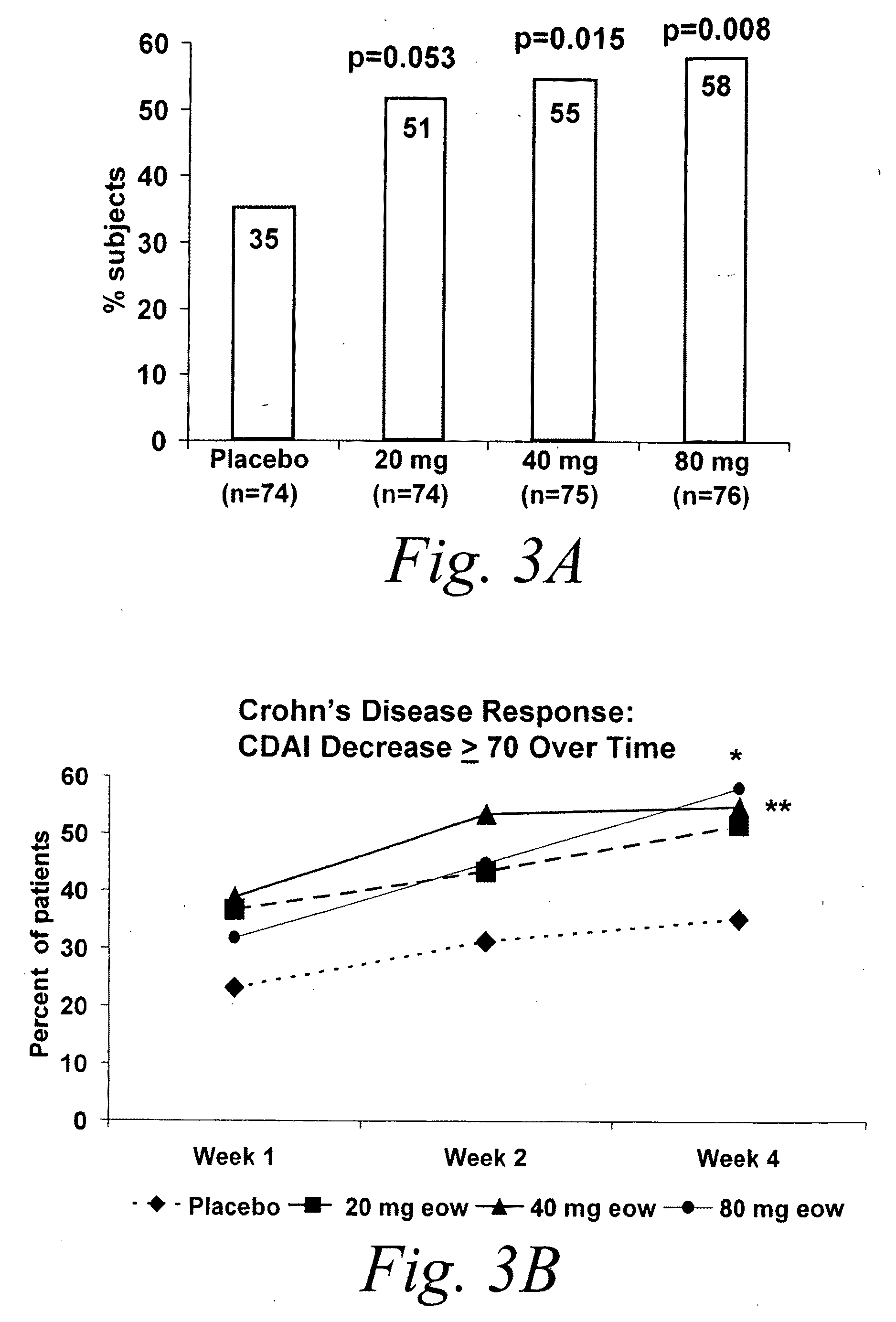
[0469]Studies were performed to determine the efficacy of a multiple-variable dose regimen of a TNFα inhibitor, namely D2E7 (also referred to as adalimumab and Humira®), for treating Crohn's disease (CD). Efficacy and tolerability of D2E7 in the treatment of patients with active Crohn's disease were evaluated in the following randomized, double-blind, placebo-controlled, multicenter study. Another objective of the following study was to assess the pharmacokinetics of adalimumab (ADA or D2E7), a fully human monoclonal antibody recognizing TNF, following subcutaneous (sc) administration in patients with Crohn's disease. To assess the pharmacokinetics of adalimumab was determined, a fully human monoclonal antibody TNF-antagonist, following subcutaneous (sc) administration over 4 weeks in patients with CD who participated in this...
example 2
Additional Study of Efficacy of Multiple-Dose Therapy for Treatment of Crohn's Disease
[0486]Multiple-Variable Dose Treatment of Crohn's Disease with D2E7
[0487]A study was performed to assess the tolerability and clinical benefit of a multiple-variable dose treatment using a TNFα inhibitor, specifically D2E7, in adult patients with Crohn's disease who had previously received and responded to a different TNFα inhibitor. The study included patients who had previously received the chimeric anti-TNF antibody infliximab, but who no longer have a sustained response and / or tolerance to infliximab.
[0488]Patients who had lost responsiveness or developed intolerance (acute or delayed infusion reactions) were treated with D2E7 80 mg at week 0 and 40 mg at week 2. All treatments were subcutaneous. Antibodies to infliximab (ATI) were determined at baseline (Prometheus Laboratories, San Diego, Calif.). Crohn's disease activity index (CDAI) scores, presence of fistulas, and C-reactive protein (CRP)...
example 3
Efficacy of Multiple-Dose Therapy Using TNFα Inhibitor for Treatment of Psoriasis
[0491]Multiple-Variable Dose Treatment of Psoriasis with D2E7
[0492]A study was performed to determine the efficacy of a multiple-variable dose regimen of D2E7 for treating psoriasis. Efficacy and tolerability of D2E7 in the treatment of patients with moderate to severe chronic plaque psoriasis were evaluated in a randomized, double-blind, placebo-controlled multicenter study.
[0493]In this study, one hundred forty-eight adult patients with a diagnosis of moderate to severe psoriasis for at least one year were selected to receive multiple-variable dose treatment. Patients were also selected based on an affected body surface area (BSA) of ≧5%. Subjects were randomized equally to one of three groups (two treatment groups and one placebo).
[0494]At baseline (Week 0) patients in both treatment groups received an induction dose of 80 mg of D2E7. Patients in the first treatment group subsequently received a trea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com