Specific fluorescence probe substrates of human carboxylesterase 2 and application thereof
A carboxylesterase and probe substrate technology, applied in the field of medicine, can solve problems such as toxic and side effects, and achieve the effects of high sensitivity, easy detection, and simple and easy synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
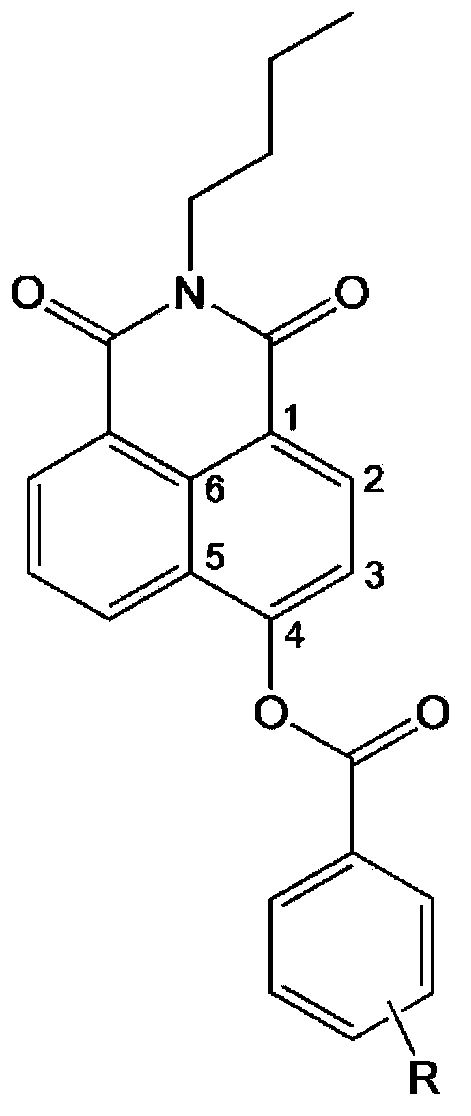
[0029] The chemical synthesis of embodiment 1.C-4 hydroxynaphthalimide benzoyl ester
[0030] (1) Slowly add 0.6 mmol of benzoyl chloride (dissolved in 5 mL of in tetrahydrofuran), the temperature was controlled at 0°C;
[0031] (2) After mixing for 1 hour, heat the reaction solution to room temperature and react overnight (see Figure 8 );
[0032] (3) The solvent was removed from the reaction solution under reduced pressure, and the residual solid was purified by silica gel chromatography, and eluted with ethyl acetate-n-hexane (1:3 v / v), to obtain 62 mg of pure white solid powder;
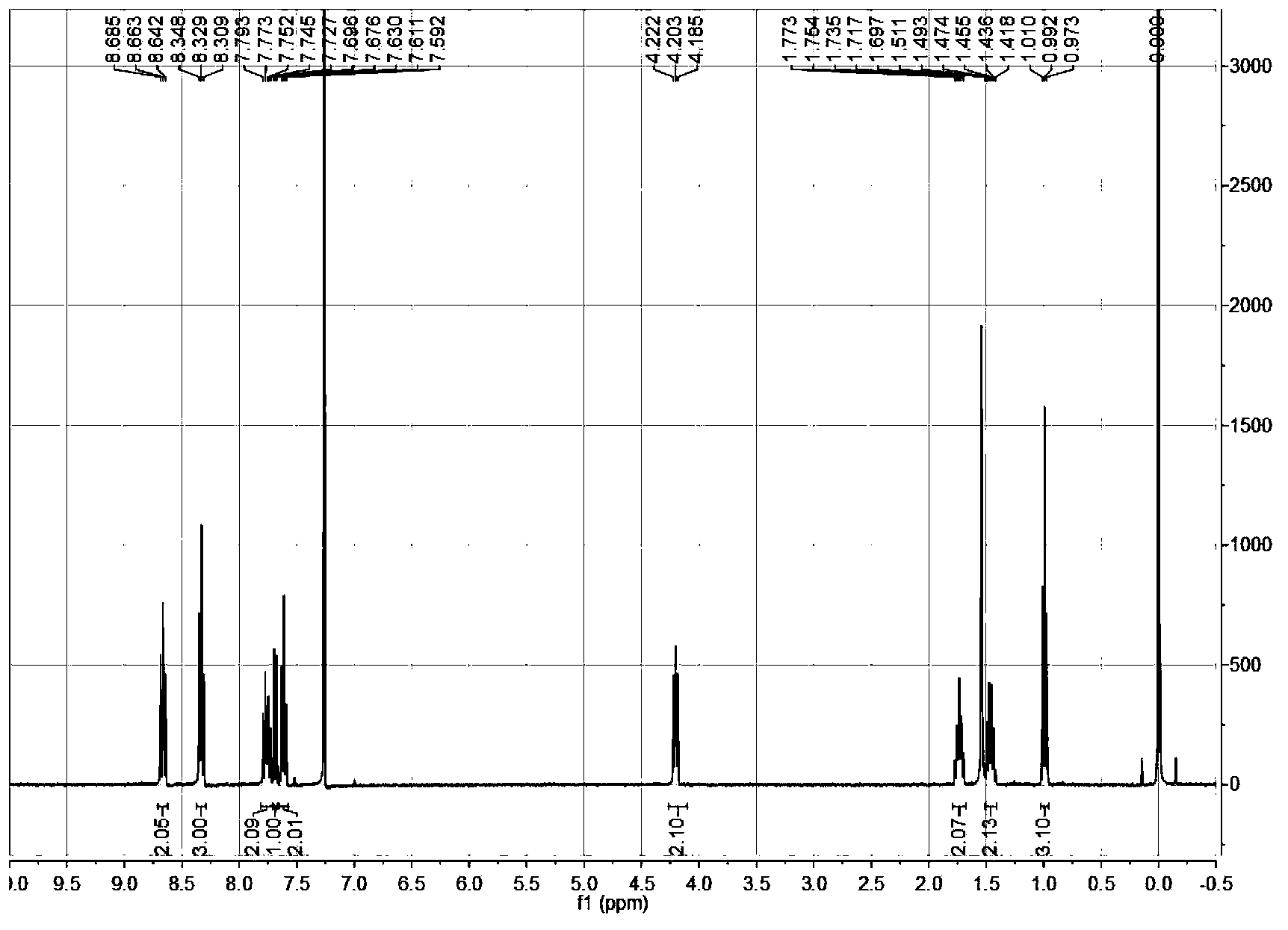
[0033] (4) Using high-resolution mass spectrometry and NMR to characterize the structure of the compound;
Embodiment 2
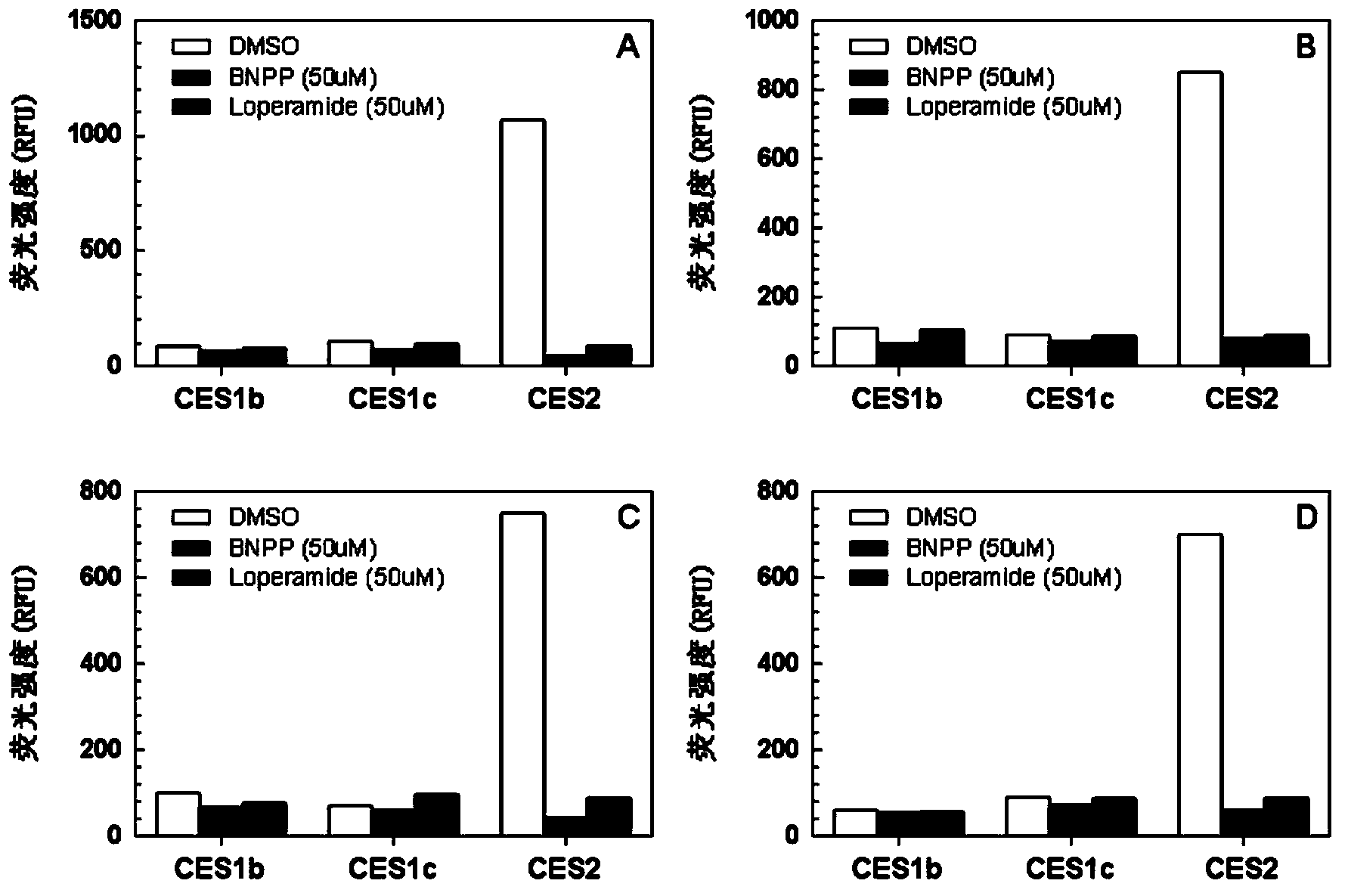
[0034] Embodiment 2. In vitro quantitative determination of the enzyme activity of CES2 in the recombinant single enzyme
[0035] (1) Prepare 99 μl CES2 metabolic reaction system in advance, including PBS buffer (10mM) at pH 7.4 and recombinant human CES2 (2ug / ml), and pre-incubate with shaking at 37°C for 10 minutes;
[0036] (2) Add 1 μl of C-4 hydroxynaphthalimide benzoyl ester with a final concentration of 25 μM to the reaction system to initiate the reaction;
[0037] (3) After 30 minutes, add 100 μl of glacial acetonitrile, shake vigorously, and terminate the reaction;
[0038] (4) Use a high-speed refrigerated centrifuge at 4°C, 20,000×g, after high-speed centrifugation for 5 minutes, take the supernatant, and perform fluorescence detection (Ex=450nm, Em=560nm); calculate the maximum catalytic activity of recombinant human CES2 enzyme The rate was 2310±350 nmol / min / mg.
Embodiment 3
[0039] Example 3. Quantitative determination of the enzyme activity of CES2 in human intestinal microsomes in vitro
[0040](1) Prepare 99 μl human small intestinal microsome metabolic reaction system in advance, including PBS buffer (10mM) at pH 7.4 and human small intestinal microsome (20ug / ml), and pre-incubate at 37°C for 10 minutes;
[0041] (2) Add 1 μl of methylbenzoyl ester of C-4 hydroxynaphthalimide at a final concentration of 25 μM to the reaction system to initiate the reaction;
[0042] (3) After 30 minutes, add 100 μl of glacial acetonitrile, shake vigorously, and terminate the reaction;
[0043] (4) Use a high-speed refrigerated centrifuge at 4°C, 20,000×g, centrifuge at high speed for 5 minutes, take the supernatant, and perform fluorescence detection (Ex=450nm, Em=560nm); calculate the probe in the human small intestine The maximum catalytic rate of the compound is 642±34nmol / min / mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com