Alpha type conotoxin peptide derivates and use thereof
A technology of peptide derivatives and conch, applied in the field of α-type conch polypeptide derivatives, can solve the problems of toxicity and side effect tolerance, poor treatment effect, etc., achieve strong analgesic activity, increase anti-enzymatic hydrolysis ability, folding Efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
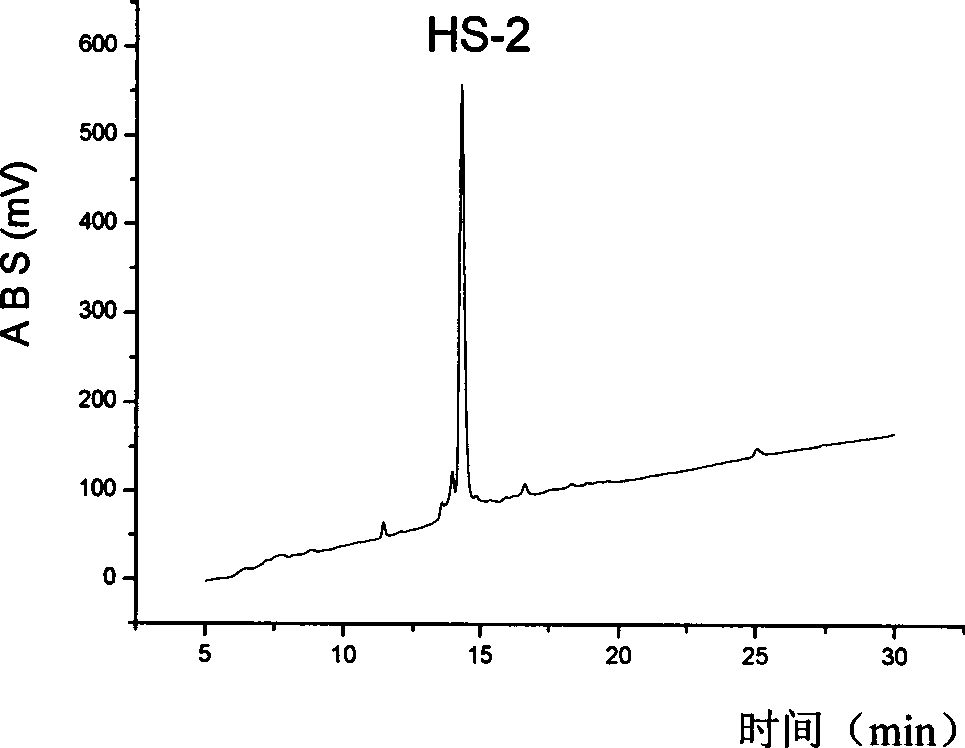
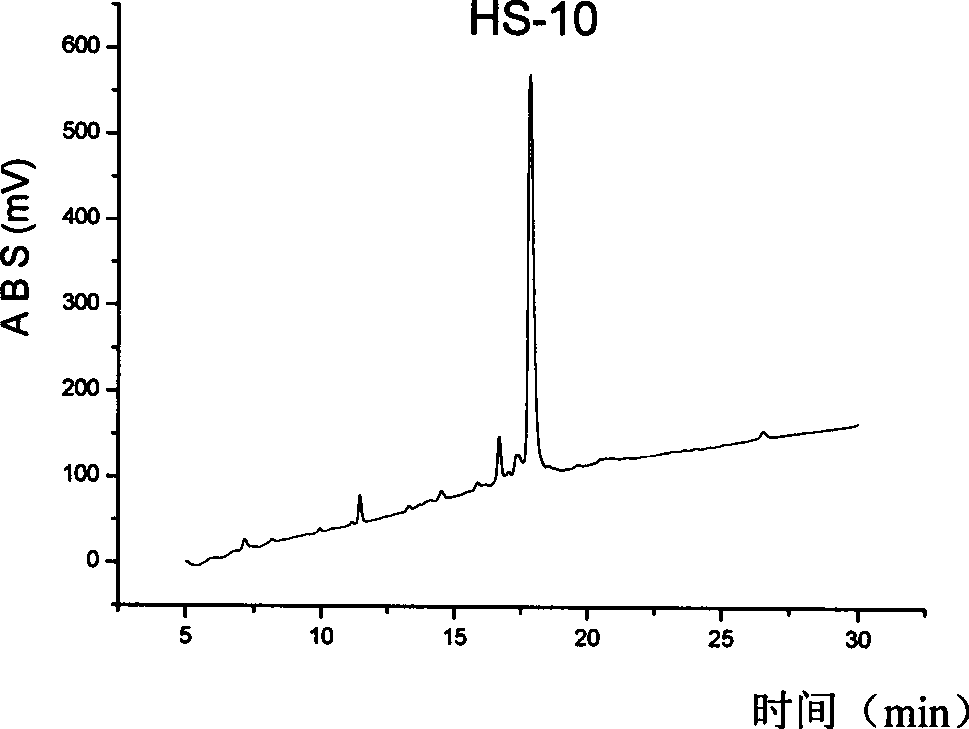
[0029] Synthesis and purification of embodiment 1, HS-2
[0030] 1. Solid phase synthesis of HS-2
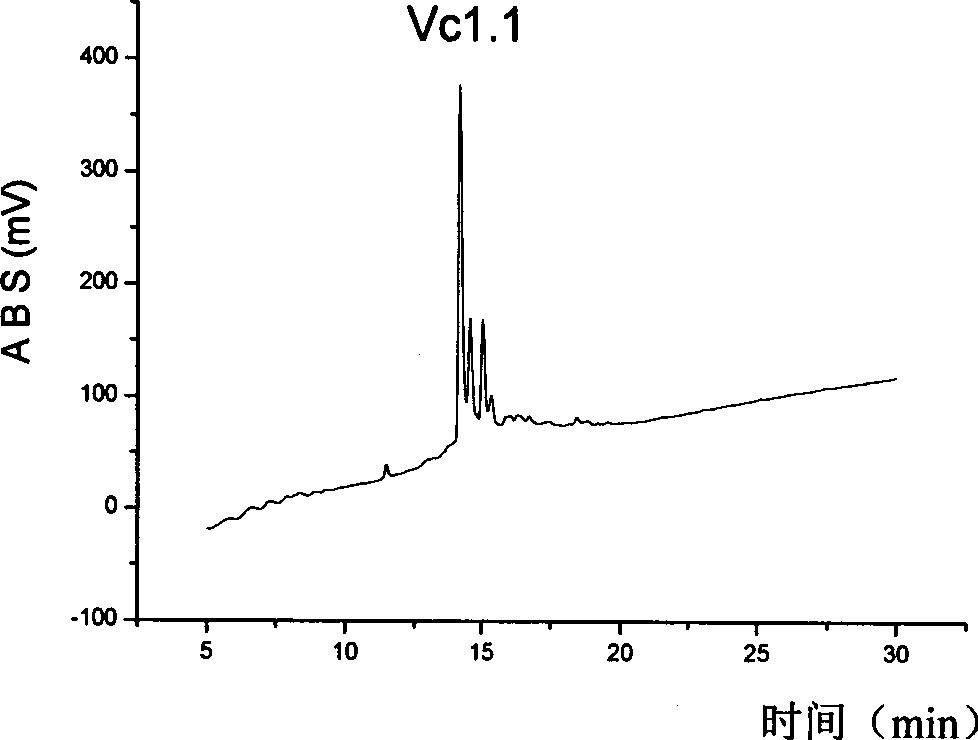
[0031] A series of α-type conopeptide derivatives were designed and synthesized, and the specific sequences are shown in Table 1.
[0032] Table 1 Sequences of α-type conus polypeptides and their derivatives
[0033]
[0034] In Table 1, A is alanine, E is glutamic acid, D is aspartic acid, C is cysteine, G is glycine, I is isoleucine, K is lysine, L is leucine Amino acid, N is asparagine, Q is glutamine, R is arginine, S is serine, V is valine, Y is tyrosine. The disulfide bond connection mode is C1-C3, C2-C4. * Indicates amidation (CONH 2 ).
[0035] The following takes HS-2 as an example to illustrate its preparation process.
[0036] 1. Synthesis of peptide resin
[0037] The synthesis of HS-2 was carried out on the 433A peptide synthesizer (ABI America Applied Systems Biological Instruments), the synthesis used solid-phase synthesis technology, and the amino acids...
Embodiment 2
[0080] Example 2: Screening α-type conus polypeptide derivatives with high analgesic activity using rat sciatic nerve hemisection model
[0081] 1. Build a model
[0082] SD rats, ♂, weighing 200-220 g, were provided by the Experimental Animal Center of the Academy of Military Medical Sciences of the Chinese People's Liberation Army.
[0083] A sciatic nerve hemisection model rat was constructed with reference to the literature (Seltzer et al. Pain, A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. 1990, 43, 205-218.). Under anesthesia and aseptic operation, the right sciatic nerve was exposed at the high position of the femur. Under a magnifying glass of 25 times, the dorsal side of the nerve was carefully separated from the surrounding tissue at the distal end of the branch of the sciatic nerve trunk to the posterior biceps and semitendinosus muscles. Separate it, clamp the dorsal side of the nerve with small hemostatic...
Embodiment 3
[0089] The dose-dependent experiment of embodiment 3, HS-2 and HS-10 analgesic effect
[0090] Seven days after the operation, the rats successfully modeled in the above-mentioned Example 2 were intramuscularly injected with the α-type conus polypeptide derivatives HS-2, HS-10 and Vc1.1 obtained in the above-mentioned Example 1. They were divided into three groups according to different injection doses: 3nmol group, 0.3nmol group and 0.06nmol group, with 6 rats in each group. At the same time, physiological saline was used as a negative control. After three consecutive days of administration, 2.5-3 hours after the third administration, the changes in the pain threshold of rats before and after administration were tested. The experiment was repeated three times, and the experimental results of the relationship between the analgesic activity and dose of HS-2 and HS-10 were as follows: Figure 4 shown. Figure 4 Among them, the ordinate is the increase rate of the pain thresho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com