SNP rs762551 of CYP1A2 gene and application thereof in relevant drug metabolism activity detection
A technology of CYP1A2 and P4501A2, which is applied in the fields of molecular biology and medicine, can solve the problems of unaffected CYP1A2 polymorphism, unclear drug metabolism activity or ability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Experimental materials and methods
[0070] 96 normal Chinese liver samples were collected clinically. The samples were collected from the pathological biopsy part of normal liver donors, about 50-200 mg per case, immediately immersed in 1 mL RNALater after collection, and kept overnight at 4°C. After the samples were fully soaked by RNALater, they were transferred to -70°C for storage. Ethics approval was obtained for the collection and use of working samples for this study.
[0071] Samples stored at -70°C were reconstituted at room temperature, removed from the RNALater, and placed in DEPC-treated ddH 2 RNALater was rinsed in O, transferred to 1 mL Trizol (Invitrogen Inc.), and homogenized with Pro200homogenizer (Pro scientific Inc.). DNA and RNA were then obtained according to Trizol's protocol. Preliminary quantification was performed with a Biophotometer (Eppendorf Inc.). The obtained RNA was treated with DNase I (Takara Inc.), purified by extraction with phen...
Embodiment 2
[0083] Validation of SNP rs762551
[0084] 2.1 DNA extraction
[0085] DNA was extracted from human blood by the conventional phenol-chloroform method, and the concentration was corrected to 20ng / ul for conventional PCR amplification.
[0086] 2.2 Design of PCR and sequencing primers
[0087] According to the sequence of CYP1A2 gene shown in SEQ ID NO:1, the following primers were designed and synthesized. The specific primers are shown in Table 1 below.
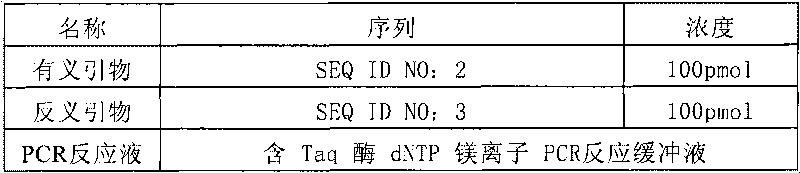
[0088] Table 1 Primer sequence list
[0089] Primer name
Sequence (5'-3')
SEQ ID NO:
sense primer
gtttgaggcc tcgggtcccc aaaggc
2
antisense primer
cagtctccac gaactcatga gtgtt
3
[0090] 1.2.3 PCR amplification of CYP1A2 gene
[0091] Using the extracted DNA as a template, PCR amplification was performed on a GeneAmp 9700 PCR instrument with the Touchdown program using Taq enzyme. The reaction conditions are: pre-denaturation at 94°C for 2 minutes, denaturation at 94°...
Embodiment 3
[0097] Individual drug metabolism activity assays
[0098] Because it is known that about 90% of caffeine is metabolized by CYP1A2 in the human body, there is a correlation between the activity of CYP1A2 and the metabolism of caffeine.
[0099] In this example, the method of Example 2 was used to genotype the CYP1A2 gene based on the SNP site at position 830 A→C in SEQ ID NO: 1 in healthy Chinese population. And observe the relationship between different genotypes and the ratio of plasma caffeine metabolism.
[0100] Select 20 healthy subjects (10 males and 10 females) aged 18-30 years old (average age 21 ± 2 years old) to participate in this test, wherein in SEQ ID NO: 1: 830 are A and C 10 each. The entire experimental process was carried out in accordance with the National Human Genome Research Ethics Guidelines, and informed consent was obtained from all subjects.
[0101] All subjects were non-smoking individuals, and had not consumed coffee, tea, Coca-Cola, chocolate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com