Protein containing serum albumin domain
a serum albumin and domain technology, applied in the direction of peptide/protein ingredients, drug compositions, antinoxious agents, etc., can solve the problems of human body, safety problems, pharmaceutical preparations using albumin derived from blood, etc., to improve the functional activity of albumin, improve the antioxidative ability, and improve the effect of albumin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
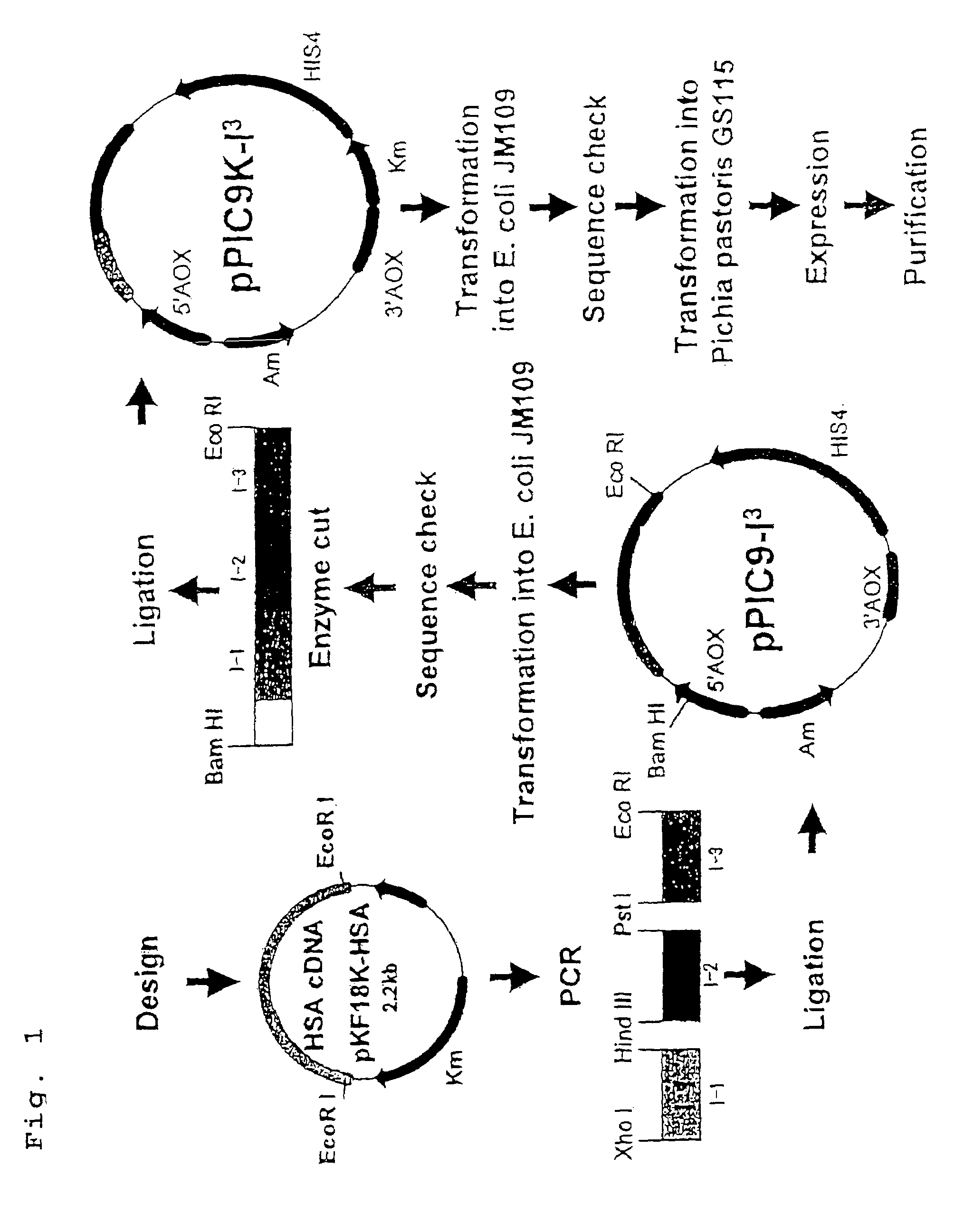
[0042]FIG. 1 shows an outline of procedures for preparing a human serum albumin domain I trimer of Example 1.
Amplification of DNA Fragment Encoding Human Serum Albumin Domain I
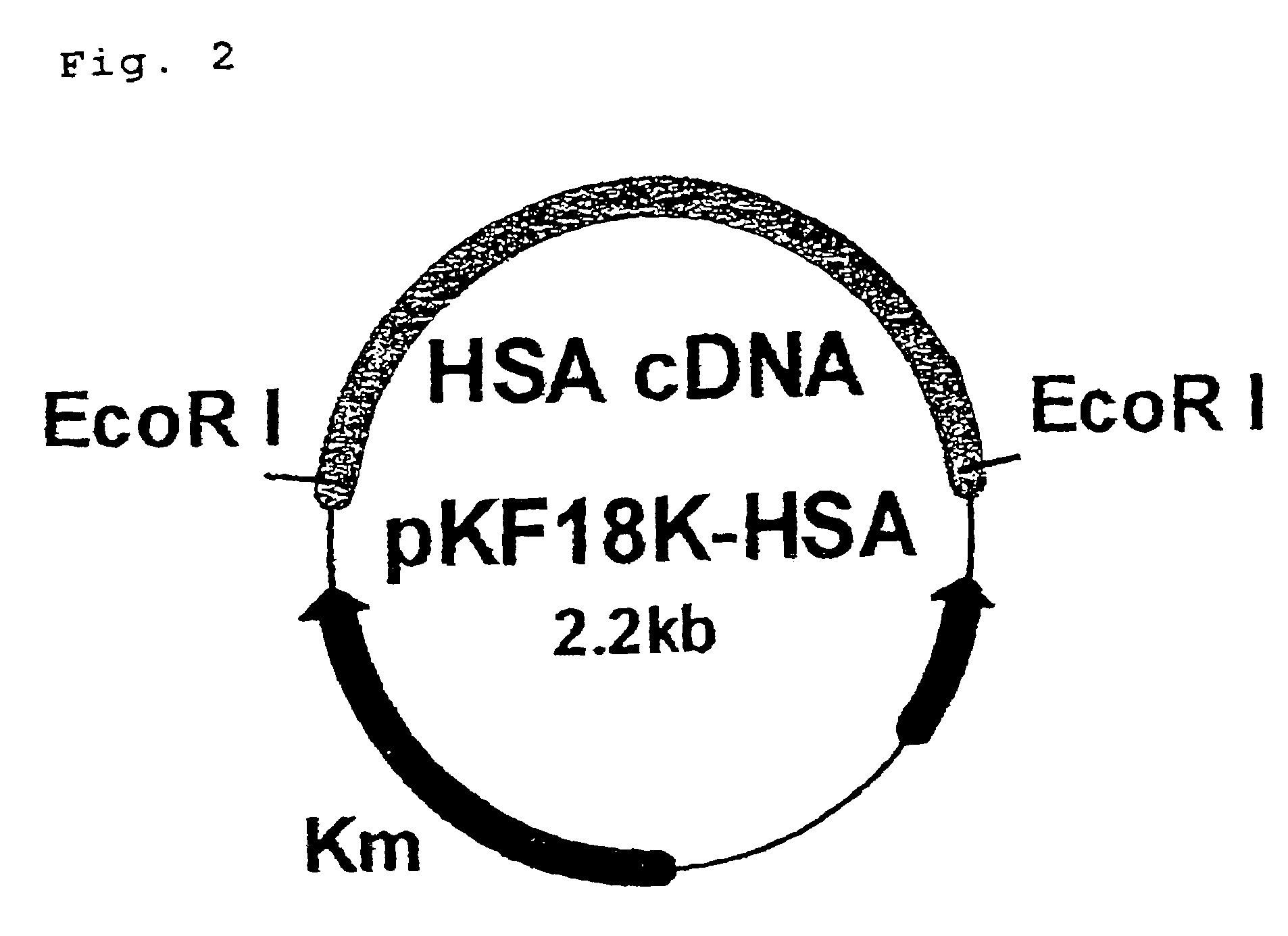
[0043]A plasmid prepared by incorporating a gene encoding human serum albumin into a plasmid pKF18K (hereinafter, pKF18K-HAS, available from TonenGeneral Sekiyu K.K.) (see FIG. 2) was used as a template. A sense primer of SEQ. ID. No. 1 and an anti-sense primer of SEQ ID NO:2, a sense primer of SEQ ID NO:3 and an anti-sense primer of SEQ ID NO:4, and a sense primer of SEQ. ID. NO:5 and an anti-sense primer of SEQ ID NO:6 were used as synthetic primers to carry out PCR using DNA polymerase (KOD-plus-, available from Toyobo Co., Ltd.). As reaction conditions for PCR, DNA was treated at 94° C. for 10 minutes, subjected to a series of reactions of denaturing (94° C., 1 min.), annealing (64° C., 1 min.), and extension (72° C., 1 min.) for 30 cycles, and then treated at 72° C. for 3 minutes. DNA fragments, to which ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| osmotic pressure | aaaaa | aaaaa |
| single stranded structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com